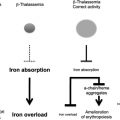
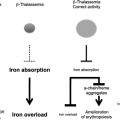
The inherited disorders of hemoglobin, including the thalassemias, are by far the commonest monogenic diseases. Although several factors are responsible for their very high frequency, the major mechanism seems to be natural selection mediated by heterozygote protection against severe forms of malaria. Recent work has highlighted the complexity of the interplay among the different hemoglobin variants themselves and among different levels of malaria resistance, and is helping to explain the extraordinary heterogeneity in the distribution of the hemoglobin disorders even within short geographical distances. Some progress has also been made toward understanding the cellular and immune mechanisms that may underlie heterozygote protection against malaria in these conditions. In addition to providing valuable information about human evolutionary biology, work in this field has an increasingly important influence on the development of programs for the better management of the hemoglobin disorders, particularly in the poorer countries of the tropical world.
The inherited disorders of hemoglobin are by far the commonest monogenic diseases. Recent estimates show that up to 350,000 babies are born each year with a serious disorder of this type, approximately 20% of which are a form of thalassemia. Approximately 90% of these births occur in low- or middle-income countries. Although the high rate of consanguineous marriage in some of these countries undoubtedly plays a role in the high frequency of births of these Mendelian recessive disorders, little doubt now exists that natural selection through heterozygote advantage against malaria is the major factor underlying their extremely high frequency and distribution.
Information collected over the past 20 years show that the high frequencies of at least some forms of thalassemia reflect heterozygote, or in some cases homozygote, protection against Plasmodium falciparum malaria, the most severe form of the disease, and some progress has been made toward understanding the cellular mechanisms involved. Recently, however, evidence has been obtained for complex epistatic interactions among the different inherited hemoglobin disorders with respect to malaria protection, at least partly explaining some profound differences in their distribution among different populations. Furthermore, it appears that some forms of thalassemia may be characterized by an increased susceptibility to infection by P vivax in early childhood. Although this form of malaria was neglected for many years and thought to be of much less clinical importance, recent work has suggested that it causes a high level of mortality and major morbidity in many parts of Asia and South America. Therefore, in addition to considerable importance with respect to explaining the current high frequency and distribution of the different forms of thalassemia, a better understanding of their interactions with different forms of malaria may have important implications for their management in the future.
This article reviews recent developments in this field and attempts to anticipate their place in future thalassemia research.
The evolution of the malaria hypothesis
Early population studies of the gene frequency for thalassemia in the Mediterranean region and United States soon raised the fundamental question as to why the condition is so high in Mediterranean populations, and not found in those of North European origin. In 1947 Neel and Valentine suggested that this might reflect variation in mutation rates between different ethnic groups. In 1949, Haldane proposed an alternative hypothesis., suggesting that, because the red cells of thalassemia carriers are smaller than normal, they might also be more resistant to attacks by the sporozoa that cause malaria, a disease that was prevalent in southern Europe until World War II. This proposal intimated that a state of balanced polymorphism exists, in which in malarious areas thalassemia carriers will tend to have more children and hence the gene frequency will increase until it is balanced by its loss in homozygotes, who die before reproductive age.
Paradoxically, shortly after Haldane’s proposal, Allison obtained independent evidence that, in the case of sickle cell disease, heterozygotes are protected against severe malaria, thus accounting for the high frequency of this condition in Africa. However, progress in determining whether Haldane’s hypothesis might be correct in the case of thalassemia was much slower. Studies in Sardinia showed that the frequency of the thalassemia gene was considerably higher in the low-lying coastal regions than in villages in the hills at an altitude at which malaria transmission does not occur. Similar evidence suggesting a relationship between the distribution of malaria and thalassemia was obtained from Italy, but when these correlations were sought in other parts of the world they were not found. All of these studies were bedevilled by problems of founder effects and gene drift, together with lack of knowledge about the origins of the thalassemia genes: had they arisen in Europe and carried east by population movement, or had they arisen in the east and moved west through a similar mechanism ? Only after it was possible to study the molecular basis for the thalassemias did it become feasible to answer some of these fundamental questions.
Thalassemia and P falciparum
α Thalassemia
The first studies in the molecular era of the interplay between thalassemia and P falciparum malaria investigated this interaction in the Southwest Pacific. Previous demographic data had shown that malaria reaches its highest frequency in Papua New Guinea and then declines in a south-easterly direction. The frequency of α + thalassemia was found to also have a clinal distribution across this region, being highest in the coastal regions of Papua New Guinea, with a carrier rate of approximately 70%, and shifting to less than 5% in New Caledonia. Of course, this slowly declining frequency of α thalassemia mirroring a fall in the frequency of malaria transmission could be explained by the gene having been introduced from the Asian mainland and carried south by the early populations that spread across this region, hence being diluted as they moved further south. However, analysis of the molecular forms of α thalassemia and their particular HBA haplotypes provided clear evidence that the form of α thalassemia in this region was different from that of the Asian mainland and that it had almost certainly arisen locally and been amplified by a locally acting mechanism, presumably malaria. The same form of α thalassemia was found in some, but not all, of the Pacific islands in which malaria had never been recorded. However, it was always the same mutation that had been found in the Vanuatu Islands and hence seemed to have been distributed around the Pacific as its islands were populated.
The causal relationship of the high gene frequencies of α thalassemia to protection against P falciparum malaria was confirmed by case-control studies in Papua New Guinea, which provided clear evidence that both heterozygotes and homozygotes for α + thalassemia are protected against the more serious complications of P falciparum malaria infection. Later, similar studies performed in Africa confirmed that α thalassemia offers considerable protection against the complications of P falciparum infection and that protection against malaria-associated anemia is also mediated in mild P falciparum infections accompanied by inflammation. In addition, an unexpected finding was that α thalassemia seems to protect children against infections other than malaria, both in Papua New Guinea and Kenya.
Several mechanisms have been described that at least partly explain the protective effect of α thalassemia against malarial infection. Studies of a large cohort of children in Vanuatu, an island with an extremely high rate of transmission of both P falciparum and P vivax malaria, suggested that babies with uncomplicated malaria and enlargement of the spleen had a higher frequency of α + thalassemia than age-matched normal babies. It was suggested that the early susceptibility to P vivax , which may reflect the more rapidly turning over population of red cells in α + thalassemic infants, may be acting as a natural vaccine by inducing cross-species protection against later P falciparum infection. Of course, this protective mechanism would only be relevant to populations in which both varieties of parasite exist.
At the cellular level, no evidence shows a reduced rate of invasion or growth of P falciparum in the red cells of individuals with α + or α 0 thalassemia. It has been found, however, that these cells consistently bind more malaria-immune globulin than normal cells. Furthermore, α thalassemic red cells infected with parasites are more susceptible to phagocytosis in vitro, and are less able than normal red cells to form rosettes, an in vitro phenomena whereby uninfected red cells bind to infected cells. Experts have observed that complement receptor 1 (CR1) expression, which is essential for rosette formation, is reduced on α thalassemia red cells, offering a plausible explanation for reduced rosetting and, because the amount of rosetting strongly correlates with the severity of malaria infection, for a protective mechanism. Furthermore, infected α thalassemic red cells are less able to adhere to human umbilical endothelial cells and are less susceptible to phagocytosis. Because rosetting and cytoadherence are mechanisms that may be involved in the sequestration of infected red blood cells, these findings together suggest that abnormalities of the α thalassemic red cell membrane may be of fundamental importance in protecting against the severe complications of malaria.
It has also been suggested that the relatively high red cell counts in heterozygotes, or particularly homozygotes, for α + thalassemia may provide a further mechanism for protection, notably against the profound anemia that characterizes severe P falciparum infection in young infants.
The mechanism involved in protection against nonmalarial infections is unclear. It could be mediated through prevention of malaria-associated immune suppression or acquisition of nonspecific immunity against malaria that also protects against other diseases.
β Thalassemia
Apart from an early case-control study in northern Liberia, which suggested that the β thalassemia trait is protective against severe malaria, no further studies of this type have been performed. However, extremely suggestive evidence shows that the β thalassemias have reached their high frequency through selection against malaria. The comparative altitude studies mentioned earlier have been confirmed, showing a higher frequency of β thalassemia in the low-lying coastal regions of Papua New Guinea compared with frequencies in the mountainous regions.
In evolutionary terms, it seems likely that P f alciparum is a fairly recent human pathogen. A finding favoring β thalassemia being a relatively recently acquired polymorphism is the fact that every population in which this disease is common has a different set of mutations. Furthermore, studies of HBB haplotypes and their relationship to thalassemia mutations has provided further information in this respect. Unlike the HBA haplotypes, a “hot-spot” exists for recombination in the HBB cluster, resulting in distinct 5′ and 3′ haplotypes. Admixture between these haplotypes seems to have occurred among human populations, but this is not observed in the case of β thalassemia. The thalassemia mutations, which occur in the 3′ haplotype, are almost always associated with the same 5′ haplotype, indicating that they arose much more recently in evolutionary terms and that there has not been time for admixture of the haplotypes that carry these genes. This theory suggests a very recent selective pressure, approximately 5000 years, which agrees with current estimations of the time that human populations have been exposed to the pathogenic forms of Plasmodium .
In vitro culture studies have shown that the rates of invasion and growth of P falciparum in β thalassemic red cells do not differ significantly from those in normal cells. However, early studies showed that parasite growth is significantly retarded in red cells that contain more than 5pg/HbF per cell, an observation that was confirmed later using a transgenic mouse model carrying human γ genes. Because good evidence shows that the rate of decline of HbF production after birth is delayed in β thalassemia heterozygotes, this could provide a mechanism of protection during the first year of life.
Hemoglobin E
Because HbE is synthesized at a slightly reduced rate, homozygotes have the hematologic phenotype of the β thalassemia trait, whereas heterozygotes have very slightly reduced red cell indices. Because this variant reaches extremely high frequencies throughout parts of India and southeast Asia, reaching up to a 70% carrier frequency in parts of northern Thailand, it is highly likely that it has been influenced by strong selection, at least at some time during human evolution.
No formal case control studies have analyzed the putative protective effect of the HbE trait against malaria. However, extended linkage disequilibrium in the region of the HBB gene carrying the HbE variant provides strong evidence that this mutation has come under intense and relatively recent selective pressure. The HbE trait was found to be significantly associated with a reduced severity of disease in adults admitted with severe P falciparum malaria. In vitro culture evidence also shows that red cells from HbE heterozygotes, although not homozygotes, are more resistant to invasion by P falciparum .
Therefore, although the evidence is still incomplete, relative heterozygote resistance against severe malaria seems to be at least one of the important mechanisms responsible for the extraordinarily high gene frequencies of this variant in many Asian countries.
Thalassemia and P falciparum
α Thalassemia
The first studies in the molecular era of the interplay between thalassemia and P falciparum malaria investigated this interaction in the Southwest Pacific. Previous demographic data had shown that malaria reaches its highest frequency in Papua New Guinea and then declines in a south-easterly direction. The frequency of α + thalassemia was found to also have a clinal distribution across this region, being highest in the coastal regions of Papua New Guinea, with a carrier rate of approximately 70%, and shifting to less than 5% in New Caledonia. Of course, this slowly declining frequency of α thalassemia mirroring a fall in the frequency of malaria transmission could be explained by the gene having been introduced from the Asian mainland and carried south by the early populations that spread across this region, hence being diluted as they moved further south. However, analysis of the molecular forms of α thalassemia and their particular HBA haplotypes provided clear evidence that the form of α thalassemia in this region was different from that of the Asian mainland and that it had almost certainly arisen locally and been amplified by a locally acting mechanism, presumably malaria. The same form of α thalassemia was found in some, but not all, of the Pacific islands in which malaria had never been recorded. However, it was always the same mutation that had been found in the Vanuatu Islands and hence seemed to have been distributed around the Pacific as its islands were populated.
The causal relationship of the high gene frequencies of α thalassemia to protection against P falciparum malaria was confirmed by case-control studies in Papua New Guinea, which provided clear evidence that both heterozygotes and homozygotes for α + thalassemia are protected against the more serious complications of P falciparum malaria infection. Later, similar studies performed in Africa confirmed that α thalassemia offers considerable protection against the complications of P falciparum infection and that protection against malaria-associated anemia is also mediated in mild P falciparum infections accompanied by inflammation. In addition, an unexpected finding was that α thalassemia seems to protect children against infections other than malaria, both in Papua New Guinea and Kenya.
Several mechanisms have been described that at least partly explain the protective effect of α thalassemia against malarial infection. Studies of a large cohort of children in Vanuatu, an island with an extremely high rate of transmission of both P falciparum and P vivax malaria, suggested that babies with uncomplicated malaria and enlargement of the spleen had a higher frequency of α + thalassemia than age-matched normal babies. It was suggested that the early susceptibility to P vivax , which may reflect the more rapidly turning over population of red cells in α + thalassemic infants, may be acting as a natural vaccine by inducing cross-species protection against later P falciparum infection. Of course, this protective mechanism would only be relevant to populations in which both varieties of parasite exist.
At the cellular level, no evidence shows a reduced rate of invasion or growth of P falciparum in the red cells of individuals with α + or α 0 thalassemia. It has been found, however, that these cells consistently bind more malaria-immune globulin than normal cells. Furthermore, α thalassemic red cells infected with parasites are more susceptible to phagocytosis in vitro, and are less able than normal red cells to form rosettes, an in vitro phenomena whereby uninfected red cells bind to infected cells. Experts have observed that complement receptor 1 (CR1) expression, which is essential for rosette formation, is reduced on α thalassemia red cells, offering a plausible explanation for reduced rosetting and, because the amount of rosetting strongly correlates with the severity of malaria infection, for a protective mechanism. Furthermore, infected α thalassemic red cells are less able to adhere to human umbilical endothelial cells and are less susceptible to phagocytosis. Because rosetting and cytoadherence are mechanisms that may be involved in the sequestration of infected red blood cells, these findings together suggest that abnormalities of the α thalassemic red cell membrane may be of fundamental importance in protecting against the severe complications of malaria.
It has also been suggested that the relatively high red cell counts in heterozygotes, or particularly homozygotes, for α + thalassemia may provide a further mechanism for protection, notably against the profound anemia that characterizes severe P falciparum infection in young infants.
The mechanism involved in protection against nonmalarial infections is unclear. It could be mediated through prevention of malaria-associated immune suppression or acquisition of nonspecific immunity against malaria that also protects against other diseases.
β Thalassemia
Apart from an early case-control study in northern Liberia, which suggested that the β thalassemia trait is protective against severe malaria, no further studies of this type have been performed. However, extremely suggestive evidence shows that the β thalassemias have reached their high frequency through selection against malaria. The comparative altitude studies mentioned earlier have been confirmed, showing a higher frequency of β thalassemia in the low-lying coastal regions of Papua New Guinea compared with frequencies in the mountainous regions.
In evolutionary terms, it seems likely that P f alciparum is a fairly recent human pathogen. A finding favoring β thalassemia being a relatively recently acquired polymorphism is the fact that every population in which this disease is common has a different set of mutations. Furthermore, studies of HBB haplotypes and their relationship to thalassemia mutations has provided further information in this respect. Unlike the HBA haplotypes, a “hot-spot” exists for recombination in the HBB cluster, resulting in distinct 5′ and 3′ haplotypes. Admixture between these haplotypes seems to have occurred among human populations, but this is not observed in the case of β thalassemia. The thalassemia mutations, which occur in the 3′ haplotype, are almost always associated with the same 5′ haplotype, indicating that they arose much more recently in evolutionary terms and that there has not been time for admixture of the haplotypes that carry these genes. This theory suggests a very recent selective pressure, approximately 5000 years, which agrees with current estimations of the time that human populations have been exposed to the pathogenic forms of Plasmodium .
In vitro culture studies have shown that the rates of invasion and growth of P falciparum in β thalassemic red cells do not differ significantly from those in normal cells. However, early studies showed that parasite growth is significantly retarded in red cells that contain more than 5pg/HbF per cell, an observation that was confirmed later using a transgenic mouse model carrying human γ genes. Because good evidence shows that the rate of decline of HbF production after birth is delayed in β thalassemia heterozygotes, this could provide a mechanism of protection during the first year of life.
Hemoglobin E
Because HbE is synthesized at a slightly reduced rate, homozygotes have the hematologic phenotype of the β thalassemia trait, whereas heterozygotes have very slightly reduced red cell indices. Because this variant reaches extremely high frequencies throughout parts of India and southeast Asia, reaching up to a 70% carrier frequency in parts of northern Thailand, it is highly likely that it has been influenced by strong selection, at least at some time during human evolution.
No formal case control studies have analyzed the putative protective effect of the HbE trait against malaria. However, extended linkage disequilibrium in the region of the HBB gene carrying the HbE variant provides strong evidence that this mutation has come under intense and relatively recent selective pressure. The HbE trait was found to be significantly associated with a reduced severity of disease in adults admitted with severe P falciparum malaria. In vitro culture evidence also shows that red cells from HbE heterozygotes, although not homozygotes, are more resistant to invasion by P falciparum .
Therefore, although the evidence is still incomplete, relative heterozygote resistance against severe malaria seems to be at least one of the important mechanisms responsible for the extraordinarily high gene frequencies of this variant in many Asian countries.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree