Through 5,000 years of practice, physicians, surgeons, clergy, or lay people have used thermal therapy to treat mass lesions now known as cancer. The methods have changed dramatically over this time span and certainly the techniques have improved the efficacy and safety. Hyperthermia used in combination with chemotherapy or ionizing radiation continues to improve outcomes. The authors briefly describe the historical role of hyperthermia in cancer care as well as modern expectations based on technological advancements. In particular, the article focuses on the role of hyperthermia for cancers that do not have other, more effective treatments.
Hyperthermia has been used with an “intent to cure” tumors for at least 4000 years, and as a tool for the destruction of tumor masses well before that. Tumors refer to any growth or mass that has developed unexpectedly. Well before there was any understanding of the molecular basis for cancer, let alone the ability to diagnose cancer, there was an understanding that cutting or burning of these lesions was an appropriate therapy for some affected individuals. In fact, Hippocrates describes that if a tumor “cannot be cut, it should burned. If it cannot be burned, then it is incurable.” However shocking, for many cancers this is still the case.
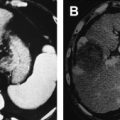
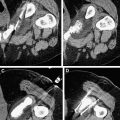
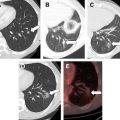
While chemotherapy may “cure” a few fortunate patients with various types of cancer, malignant diseases such as metastatic hepatocellular carcinoma (HCC) are usually incurable, with no meaningful 5-year survival probability in the majority of patients. In other patients who have locally advanced unresectable hepatic lesions, radiofrequency (RF) thermal ablation is a useful and potentially curative therapy for HCC. Notwithstanding the few patients who have some benefit from transhepatic arterial embolization, there is no curative systemic or regional cytotoxic chemotherapy for HCC. The most recently approved targeted therapy for HCC, sorafenib, increases median survival of patients with unresectable HCC by 3 months. Patients with unresectable metastatic lesions (such as colorectal cancer) to the liver that are amenable to RF ablation have a median overall survival of 25 to 30 months, in general.
There are multiple forms of hyperthermic therapy. The aforementioned RF ablation technique is a local therapy involving intratumoral placement of a needle electrode that can produce tissue temperatures as high as 100°C following activation of an electrical current. Alternating electrical current dissipation and ionic stimulation within tissue surrounding the electrode causes hyperthermia. Regional hyperthermia has been used in combination with regional chemotherapy during resection of extremity soft tissue sarcomas or as treatment for in-transit limb metastases from melanoma. In these cases, the elevated tissue temperature is maintained for extended periods of time. This procedure takes the form of an isolated limb perfusion of chemotherapy warmed to 42°C to 45°C for 60 minutes or longer. Likewise, hyperthermic intraperitoneal chemotherapy is another regional hyperthermic treatment often performed simultaneously with resection of peritoneal malignant disease. Finally, whole body hyperthermic therapy has been used by inducing fevers with toxins or externally warming entire patients up to 42°C for extended periods of time.
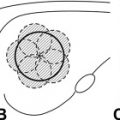
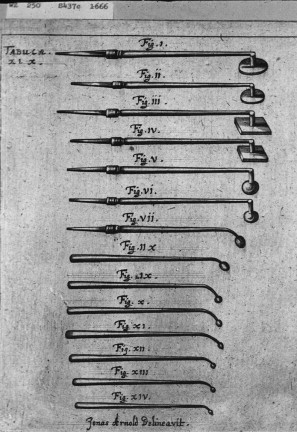
It is often quoted that Hippocrates “managed” superficial tumors with cautery or direct ablative therapy, but it is not clear if he was actually describing the treatment of cancer. It is interesting that the side effects he mentions include weakness, neurologic changes, hemorrhage, and death, which are all adverse events that are similar to what is seen today with whole body hyperthermic treatment. Around the Middle Ages and later, instruments were designed and shaped for direct application of heat to kill tumors or cauterize bleeding, albeit without the benefit of adequate regional or general anesthesia ( Fig. 1 ).
Finally, the goal of hyperthermic therapy is to capitalize on the difference in thermotolerance between normal and cancer cells. Mammalian cells die when exposed to temperatures above 55°C for more than a few minutes; however, these normal cells tolerate temperature ranges from 41°C to 43°C for hours. Of importance, each half of a degree increase in cellular temperature is associated with increased cell death. Cancer cells in general do not tolerate these temperatures nearly as well or as long. Finding the appropriate balance of temperature and duration is finding the balance between the desired cancer cell death and undesirable normal cell toxicity.
Early electrosurgery
Electrosurgical procedures in the first half of the twentieth century included destruction of cancerous tissues, enlarged lymph nodes, and cauterization of nodules left after enucleation of other masses. Of note, these included intra-abdominal procedures of the uterus and ovaries as well as intrathoracic procedures for cancers and infections. In 1900, the first modern example of curative electrosurgery for cancer was documented when an artist who had a cutaneous carcinoma accidently touched an electrical wire. The current “treated” his cutaneous carcinoma via hyperthermia, and the concept of electrofulguration was born. Soon thereafter, William T. Bovie and Harvey Cushing developed and clinically implemented an electrosurgical device for decreasing intraoperative blood loss. Hyperthermic electrothermal ablation can directly trace its history to this moment.
Development of modern techniques
Modern ablative techniques require direct contact between a probe, the target tumor, and surrounding normal tissue. Depending on the modality and intensity of treatment, there is an immediate zone of intratumoral necrosis, a zone of apoptosis, and a zone of hyperemia without frank cell death. Ideally, there will be a margin of normal tissue death to ensure the death of all cancer cells. Among many extremely well-conducted studies and reviews during the 1970s investigating the effects of hyperthermia on normal and cancer cells, a review article by Field and Bleehen described the current understanding of hyperthermia in the treatment of cancer that remains accurate to this day.
Effective local or regional hyperthermic cancer therapy can be induced by either longer heating durations at temperatures of 41°C to 45°C or by short-duration treatment of cancer cells with higher temperatures (or both). Likewise, many, but not all, cancer cells respond differently from their normal cell origins to hyperthermic therapy. The difference between these 2 responses permits a general method to treat cancers with hyperthermia. This is not to suggest that all cancers can be treated in the same manner, but this approach has worked well in RF ablation of primary and secondary liver malignancies. The approach is to maximize the coagulative necrosis of the malignancy in situ while accepting limited necrosis or apoptosis of surrounding normal parenchyma. In this way, excess normal tissue is not needlessly injured while “oncologically” safe margins are maintained. Furthermore, all cancer cells are presumed to be within the area of coagulative necrosis and are immediately killed during the procedure. The balance of treatment effectiveness, patient safety, and normal hepatocyte tolerance resulted in a nearly uniform practice of treating properly selected hepatic tumors for approximately 10 minutes with tissue temperatures of at least 60°C to 65°C.
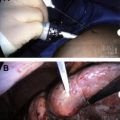
More recent examples of direct electrosurgery are seen in the practice of endobronchial procedures and cervical lesions. Whereas loop electrosurgical procedures are standard therapy for premalignant cervical lesions, at present endobronchial ablative therapy is not standard for cancers of the upper airways. However, it provides interesting and effective use of hyperthermic cautery based on the principles previously described. Of note, complete excision of premalignant or early malignant cervical lesions confers extremely high rates of cure for a disease, much like HCC, that otherwise carries a very poor prognosis when it is found to be advanced.
Development of modern techniques
Modern ablative techniques require direct contact between a probe, the target tumor, and surrounding normal tissue. Depending on the modality and intensity of treatment, there is an immediate zone of intratumoral necrosis, a zone of apoptosis, and a zone of hyperemia without frank cell death. Ideally, there will be a margin of normal tissue death to ensure the death of all cancer cells. Among many extremely well-conducted studies and reviews during the 1970s investigating the effects of hyperthermia on normal and cancer cells, a review article by Field and Bleehen described the current understanding of hyperthermia in the treatment of cancer that remains accurate to this day.
Effective local or regional hyperthermic cancer therapy can be induced by either longer heating durations at temperatures of 41°C to 45°C or by short-duration treatment of cancer cells with higher temperatures (or both). Likewise, many, but not all, cancer cells respond differently from their normal cell origins to hyperthermic therapy. The difference between these 2 responses permits a general method to treat cancers with hyperthermia. This is not to suggest that all cancers can be treated in the same manner, but this approach has worked well in RF ablation of primary and secondary liver malignancies. The approach is to maximize the coagulative necrosis of the malignancy in situ while accepting limited necrosis or apoptosis of surrounding normal parenchyma. In this way, excess normal tissue is not needlessly injured while “oncologically” safe margins are maintained. Furthermore, all cancer cells are presumed to be within the area of coagulative necrosis and are immediately killed during the procedure. The balance of treatment effectiveness, patient safety, and normal hepatocyte tolerance resulted in a nearly uniform practice of treating properly selected hepatic tumors for approximately 10 minutes with tissue temperatures of at least 60°C to 65°C.
More recent examples of direct electrosurgery are seen in the practice of endobronchial procedures and cervical lesions. Whereas loop electrosurgical procedures are standard therapy for premalignant cervical lesions, at present endobronchial ablative therapy is not standard for cancers of the upper airways. However, it provides interesting and effective use of hyperthermic cautery based on the principles previously described. Of note, complete excision of premalignant or early malignant cervical lesions confers extremely high rates of cure for a disease, much like HCC, that otherwise carries a very poor prognosis when it is found to be advanced.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree