Fig. 1.1
Diagram of the human airway system . There are 23 generations of branching airways on average, beginning with larger airways in the conducting zone, consisting of the trachea , bronchi (BR), bronchioles (BL), and finally terminal bronchioles and transitional bronchioles (TBL). The acinar airways begin to appear following the TBL, with respiratory bronchioles (RBL), alveolar ducts (AD) that terminate into alveolar sacs (AS). (Used with permission from Weibel ER: Morphometry of the Human Lung. Heidelberg: Springer-Verlag; 1963)
Alveoli begin to appear within the walls of the transitional bronchiole , and gradually increase in number in the next several generations of airway branching, i.e., respiratory bronchioles, before alveolar ducts appear and terminate into alveolar sacs. The boundary between respiratory bronchioles and alveolar ducts is somewhat arbitrary, as it relies on the relative degree of “alveolarization.” An airway is considered an alveolar duct when alveoli completely cover the airway [7]. The last eight generations of airways are together referred to as the acinus. In the human lungs, there are approximately 30,000 acini, with an average volume of 187 mm3 (SD: ± 79 mm3) [8]. These acini comprise a total of approximately eight million end branches, also known as alveolar sacs, and these alveolar sacs finally connect to approximately 300 million alveoli. Contrary to the common model of “parallel alveoli,” where all the alveoli are arranged at the end of alveolar sacs, alveoli actually start to appear in the last six to nine generations of airways [9, 10].
When comparing airway structures between different species, the most noticeable difference lies in the number of terminal bronchioles, which is the result of differences in generations of airway branching. This is largely determined by the size and shape of the thoracic cavity [1]. Another difference is the presence or absence of respiratory bronchioles [7]. In humans, there are approximately 1–3 generations of respiratory bronchioles before the appearance of alveolar ducts. However, rats and mice, which represent common animal model systems, possess only either one generation of respiratory bronchioles or are absent [7]. When these animals are used to study human diseases such as cigarette smoke-induced emphysema, which are presumed to originate in the respiratory bronchioles [11], one needs to consider anatomical differences of the model system.
Effective gas exchange depends on the large surface area of the alveolar membrane. This is made possible by arranging alveoli closely along the alveolar ducts, allowing for a large number of alveoli within the relatively confined space of the thoracic cavity. Another advantage of having this arrangement is to minimize the distance for oxygen molecules to diffuse before coming into contact with the alveolar membrane. In fact, the average longitudinal distance from transitional bronchiole to the alveolar sacs, is approximately 8.3 mm (SD: ± 1.4 mm) [8, 10].
Features of the Blood Vessel Tree
As the airway tree branches, so does the vascular tree. The pulmonary artery follows the airway tree closely, although the arterial vascular tree branches more frequently than the airway [3]. Compared with an average of 23 airway generations, there are approximately 28 generations of the pulmonary arterial branching within the human lung [3]. The pulmonary artery begins as a single trunk, branches into 70 million pre-capillary arterioles, and terminates into approximately 280 billion capillary segments [12]. At any given moment, there are approximately 200 ml of capillary blood volume bathing the entire alveolar surface [12].
As the artery branches, the accessory structures of the vessel wall also change. The walls of the major pulmonary arteries consist of a large number of collagen and elastic lamina, so they can sustain the pressure generated by the cardiac output from the right ventricle [13]. Vessels surrounded by smooth muscle fibers begin to appear when the vascular diameter falls below 1 mm [13]. The smooth muscle fibers enable these small arteries to regulate blood flow via constriction and dilation [13]. As the pulmonary artery continues to branch, the muscular wall gradually tapers until there is only one layer of smooth muscle, marking the appearance of arterioles [14]. Eventually, the muscular wall becomes incomplete, and this defines the pre-capillary region [14]. In general, the amount of smooth muscle along the pulmonary arterial wall is less compared with systemic arteries, which contributes to the low resistance of the pulmonary artery tree [1]. In the more peripheral region of the human lung, the pulmonary vein travels closely with the artery and airway within the bronchovascular bundle [13]. In the more central region of the lung, the veins travel separately to the hilum from the artery and airway [13]. For cow, pig, and sheep lung, the pulmonary veins travel along with the airway and artery within the bronchovascular bundle. For smaller mammals such as rabbit, guinea pig, and rat, the pulmonary veins course independently from the airway–artery bundle [15, 16]. Compared with the capillary networks of other organ systems, the structure of the pulmonary capillary network is quite unique. Rather than an individual capillary unit interacting with a discrete alveolar unit, pulmonary capillaries are spread across the alveolar surface of multiple alveolar units [12]. This structure of the pulmonary capillary network maximizes the contact surface area between alveolar membrane and the capillary blood for oxygenation, and minimizes the extent of shunting [10].
Bronchial Artery System
The bronchial artery system normally accounts for ~1 % of cardiac output, and mainly functions to deliver nutritive support to the large airway and vessels under basal conditions [17]. At the level of alveoli and respiratory bronchioles, bronchial arteries form connections with the pulmonary arteries via microvascular anastomoses, and create physiological shunts in the lung, as bronchial arteries do not participate in gas exchange [18]. The bronchial artery system is also a high-pressure system compared with the pulmonary arterial system [17]. Thus, when pathological changes occur in the pulmonary artery, increased bronchopulmonary collaterals and collateral blood flow may form, diverting more blood from the cardiac output through bronchopulmonary shunts, and, if significant, compromise blood oxygenation [19]. Some conditions that are associated with bronchial artery dilatation include bronchiectasis, primary pulmonary hypertension, lung cancer, Takayasu’s arteritis , and chronic thromboembolic disease [1, 18]. In some extreme disease states such as in chronic thromboembolic disease, the percentage of cardiac output circulating through bronchopulmonary collaterals may increase by 18–30 % [20, 21].
Convection–Diffusion in Airway Conductance
Oxygen transport through the large airways occurs by convective, or bulk, flow [3]. As airway branches, the diameter of the airway decreases, but the degree of airway narrowing is not uniform [3]. The degree of airway narrowing in successive generations (i.e., the more peripheral regions of the lung) is less than that of the airway located in the central regions of the lung. As previously mentioned, the transition point where this occurs is at the level of the transitional bronchiole. In fact, the airway diameters of respiratory bronchioles and alveolar ducts change very little. Given that the number of airways doubles after each generation, the total cross-sectional area of the respiratory bronchioles and alveolar ducts rapidly increases. With a given volume of gas, the oxygen bulk flow will therefore decrease, to a point where it is slower than the rate of simple diffusion [22]. After this point, diffusion becomes the predominant process. This structure–function relationship is ideal, because the airway terminates into blind ends, and the only way oxygen is delivered to the peripheral lung is by diffusion [1]. In human lungs, at rest, the convection–diffusion transition point occurs at approximately the 14–16th generation of airway branching, which corresponds roughly to the transitional bronchiole or the first generation of respiratory bronchioles [3]. The diffusion-dominant zone in human lung at rest starts at approximately generation 16 [3]. During exercise, the point of convection–diffusion transition will move more peripherally, as the initial bulk flow (convection) velocity can increase by tenfold [1, 9, 10, 23].
Interdependence of Alveolar pO2 , and Independence of Arterial pO2
In addition to the large contact surface area between the alveolar membrane and capillary blood, another important determinant of gas exchange is the pressure gradient of oxygen between alveolar (PAO2) and the capillary blood (PaO2) [1]. This pressure gradient is maintained by effective ventilation-diffusion in the airway to ensure high alveolar oxygen pressure, and sufficient capillary perfusion to supply deoxygenated blood that provides a lower capillary oxygen partial pressure [1, 4].
The acinar capillaries are arranged in parallel, so for each capillary unit, its oxygen partial pressure will be the same, and is independent of other capillary units [3]. On the contrary, alveoli first appear within the walls of the respiratory bronchiole, and are arranged in series along the acinar airway [3]. Given the finite amount of oxygen in each breath, the alveoli that are close to the entrance of the acinar airway will be ventilated first. As the oxygen diffuses across the blood-gas membrane of these proximal alveoli, the amount of oxygen that is left decreases, and therefore the alveolar oxygen partial pressure decreases as it moves towards more peripheral alveoli [23]. In other words, the PAO2 is interdependent between the proximal and distal alveoli [23]. About half the surface area of the gas-exchange membrane of an acinar airway is located in the last generation. If the gas-exchange membrane is too permeable to oxygen, then by the time oxygen reaches the last generation of airway, the PAO2 will be so low that little oxygen can diffuse across the membrane, and the peripheral gas-exchange surface is underutilized, or “screened,” for diffusion [23]. If oxygen permeability of the alveolar membrane is too low, oxygen will require a much larger total surface area for gas-exchange to sustain metabolic activity of the organism, and this will require a larger thoracic cavity size [23]. With the convection–diffusion transition point moving more distally during exercise, less alveolar surface area is screened for diffusion, compared with the area being screened when the subject is at rest [23]. By mathematical modeling, others have shown that in human lungs, the relationship between oxygen permeability across the gas-exchange membrane and oxygen diffusion capacity in the distal airways provides functional reserve for oxygenation while avoiding strong diffusion screening at rest [9, 10, 23].
Oxygen Diffusion Capacity
The capacity of oxygen molecules to travel from alveolar air to red blood cells is called the oxygen diffusion capacity. It is comprised of two serial events, the first of which is the oxygen transfer across the blood-gas membrane by passive diffusion. The second event is the binding of oxygen to hemoglobin [1]. The surface area available for gas exchange, the extreme thinness of the membrane structure, and the oxygen partial pressure gradient across the alveolar and capillary compartment enhances diffusion capacity [24]. The binding of oxygen molecules to hemoglobin is determined by the biochemical properties of the hemoglobin, as well as the content of total hemoglobin that is available for oxygenation. Clinically, the oxygen diffusing capacity is reflected by the diffusing capacity of the lungs for carbon monoxide (DLCO) [25]. Carbon monoxide is chosen as the testing agent because of its high affinity to hemoglobin, and therefore the test result will not be sensitively affected by changes in cardiac output [25]. DLCO is the product of two measurements, the first measurement of which is the rate constant (KCO), reflecting the rate of CO uptake from alveolar gas, and the second is the alveolar volume (VA). KCO is, by definition, a direct characterization of the diffusion capacity through the gas-exchange membrane and the binding of hemoglobin [25]. KCO is reduced when there is pulmonary capillary destruction (i.e., pulmonary vasculitis), remodeling, and dilatation (i.e., pulmonary arteriovenous malformation), or alveolar destruction (such as emphysema), and increased when there is increased pulmonary blood volume (as seen in alveolar hemorrhage, congestive heart failure, left-to-right intracardiac shunts), or with reduced alveolar expansion (such as respiratory muscle weakness or pneumonectomy) [26]. The passage for CO2 is similar but in reverse direction. Because CO2 is more readily capable of diffusing across the blood-gas membrane than O2, the exchange of CO2 will not be impaired as long as there is enough ventilation [1].
Red Blood Cell as Vehicles of Gas Exchange
The principle carrier of oxygen in the blood is hemoglobin, which transports approximately 20 mL of oxygen per 100 mL of blood [4]. A small portion of oxygen (0.3 mL/100 mL) is directly dissolved in blood [4]. As the fraction of inspired oxygen (FiO2) increases from 0.21 to 1.0, the oxygen partial pressure increases accordingly, and more oxygen is directly dissolved in the blood. When breathing in FiO2 of 1.0, the directly dissolved oxygen reaches 2 mL/100 mL, and constitutes a more significant portion of the total oxygen carrying capacity of blood [4]. Hemoglobin A, the most prevalent hemoglobin in humans, is comprised of four globin proteins, two alpha and two beta globin subunits (α2β2), noncovalently bound to each other to form a tetramer [27, 28]. A pocket is created within each globin subunit that contains heme, an iron-containing porphyrin, that binds molecular oxygen when iron is in the ferrous state [4]. The relationship between oxyhemoglobin, or the total % hemoglobin saturated with oxygen, and the partial pressure of oxygen follows a nonlinear sigmoidal-shaped curve [4]. This oxyhemoglobin disassociation curve reflects cooperative interaction between the four oxygen-binding sites resulting from conformational change of the hemoglobin structure upon binding the initial oxygen molecule that increases the affinity of hemoglobin for subsequent oxygen molecules until all four binding sites are saturated [28, 29]. The oxyhemoglobin disassociation curve encapsulates the essential function of red blood cells, that is, oxygen binding and ability to unload as a function of the partial pressure of oxygen. Hemoglobinopathies such as sickle cell anemia are single-gene mutations that lead to abnormal hemoglobin structure and can show altered oxyhemoglobin dissociation curve [30]. In sickle cell disease, sickling occurs under conditions of hypoxic stress [30]. As discussed in subsequent chapters, vaso-occlusive crisis due to the sickling of red blood cells within the pulmonary microvasculature contributes to the acute chest syndrome in sickle cell disease (see Chap. 3).
Ultrastructure of the Alveolus
There are several distinguishable cell populations comprising the alveolus, including type I alveolar epithelial (AE1), type II alveolar epithelial (AE2), and lung fibroblasts [5]. AE1 is flat and elongated, and is the main structural barrier for gas exchange together with the endothelial cell. AE2 cells are smaller and cuboidal in morphology, serve as the alveolar stem cell, and actively carry out metabolic and secretory functions, as well as immune defense, the latter function also modulated and executed by resident mononuclear phagocytes [31]. Finally, lung fibroblasts help maintain the alveolar structure by secreting extracellular matrix proteins [1, 32].
Type I Alveolar Epithelial Cells
The three determinants of effective gas exchange are (1) the large surface area of contact, (2) oxygen partial-pressure gradient across the alveolar and capillary blood, and (3) permeability of the airway-blood barrier [1]. As previously mentioned, the mammalian lung achieves the large surface area of gas exchange by generations of branching airways that end in alveoli [5]. A strategically located convection–diffusion transition point maintains an oxygen pressure gradient, as do ample amounts of deoxygenated blood coursing through the pulmonary vascular beds [23].
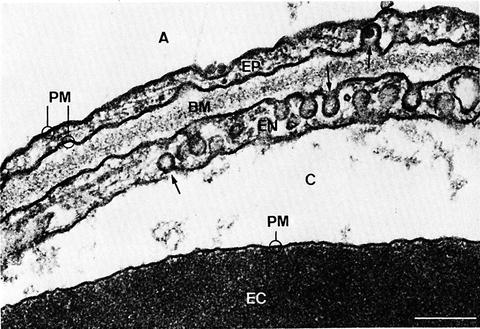
The gas-exchange membrane is comprised of the AE1 cells, capillary endothelial cell, and a fused basement membrane [5, 12], as shown by the electron microscopy image in Fig. 1.2. The blood-gas barrier is notable for its extreme thinness (~0.3 μm in total thickness in mammalian lung [33]), in part, due to the highly specialized morphology of the AE1 cells. AE1 cells only comprise 8 % of the total cells in the normal adult human lung, but covers 95 % of the alveolar surface [32]. If we divide the total alveolar surface area by the number of AE1 nuclei, the surface area covered by one type I epithelial cell is approximately 4000–5000 μm2, which is larger than what is observed under the microscope of any single AE1 cell surface [34]. This is because the Type I alveolar epithelial cell is not a simple squamous cell, but branched cells with multiple apical faces, and its cell body is spread out over several vast surfaces with minimal cytoplasm in between, except in regions near the nuclei [34]. These regions correspond respectively to the observed thin portions of the gas-exchange membrane, comprising approximately 50 % of the total surface area. In the thick portions of the blood-gas barrier, elastic fibers, collagen fibrils, and fibroblasts can be observed [1, 4]. The research on AE1 has been challenging, due to its “flatness” in morphology, and the difficulty in separating intact AE1 cells from the rest of the lung tissue.