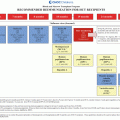
Fig. 1.1
Major transitions associated with cancer treatment. Reproduced with permission from MacLean et al. Cancer 1996; 78(6)
For some patients, transition may also include development of relapse, and, for too many, end-of-life care. Effective management of these cancer-related transitions requires strong communication skills and anticipatory guidance born of familiarity with the underlying cancer and treatment regimen, as well as the typical clinical course.
In pediatric and adolescent oncology, however, these transitions do not occur in isolation, but rather against a backdrop of the patient’s normal physical, emotional and social development. The successive transitions of developmental maturation that begin during infancy and continue through older adolescence not only influence each patient’s response to cancer-related transitions, but also cause patients to require support during the cancer experience in order for healthy adulthood to be achieved.
One additional transition of survivorship, which arguably represents a unique convergence of both cancer-related and normal developmental components, is the one which occurs between older adolescence and young adulthood. In no other transition do we encounter the simultaneous complexities of established treatment-related health problems, emerging risks, need for ongoing medical surveillance, change from pediatric to adult-focused health care services, threats to maintaining health insurance, completion of formal education, entrance to the work force, the achievement of personal independence, and redefinition of familial and societal roles, to name a few. With more and more young people surviving childhood cancer than ever before, the need for workable approaches to health care transition in young adulthood has never been greater.
The purpose of this chapter is to provide an overview of the major transitions experienced by children and adolescents undergoing management of their cancer, especially during the long-term follow-up phase. Key issues and potential interventions for each are discussed. The principles and practice of health care transition for young adult survivors of childhood and adolescent cancer are emphasized.
1.2 Developmental Aspects of Transition
Cancer commonly affects growth and development, either directly in physical changes or through parenting and peer experiences. Physicians and other health care professionals taking care of childhood cancer survivors should have an understanding of major developmental tasks of childhood in order to normalize the cancer experience in an age-appropriate way [2, 3]. Providing appropriate support for those tasks differs somewhat according to the type of transition. As summarized in Table 1.1, during transitions associated with diagnosis and treatment, the focus for all age groups is to support patients and families through crises characterized by sudden and dramatic change, unfamiliar situations, uncertain outcomes and frightening possibilities.
Table 1.1
Developmental stages of childhood and their correlates for transitional care
Developmental stage | Selected developmental features [3] | Correlates for transitional care | |
|---|---|---|---|
Diagnosis and treatment phase | Survivorship phase | ||
Preschool (2–5 years) | Acquisition of language and motor skills | Arrange child life interventions to minimize procedure-related anxiety | Child too young to understand the need for long-term follow-up |
Formation of simple concepts of reality | Facilitate child’s understanding that illness is not a punishment | Direct anticipatory guidance about late effects towards the parents | |
Emotional connection with other people | Advise parents that being calm may be more comforting than explanations like “this will make you better” | Mention eventual transition to adult-focused providers | |
Cognitive features of magical thinking, egocentrism and dominance of perception | |||
Middle childhood (6–12 years) | Expansion of child’s world outside the home | Provide simple explanations to child regarding diagnosis and necessary treatments | Provide simple explanation to child relating prior illness to the need for continued follow-up |
Ability to get along with other children | Maintain educational progress through hospital-based school activities and school reentry programs | Continue to educate parents on late effects, health and wellness | |
Development of concrete operational thinking | Encourage child’s involvement in simple treatment choices (e.g., flavor of medications) | Mention eventual transition to adult-focused providers | |
Acquisition of adult concepts and communication (writing, reading, calculating) | Advise parents against over protectiveness and encourage normal disciplining | Advise parents against over protectiveness and encourage normal disciplining | |
Encourage parents to allow children to have increasing responsibilities at home and an increasing role in decisions | |||
Early adolescence (10–13 years) | Development of formal logical operations | Provide straightforward but more detailed explanations of diagnosis and treatment | Provide straightforward but more detailed explanations about follow-up care |
Awareness of changing body and interest in opposite sex | Provide support to reduce social isolation and depression through interventions such as child life therapy | Encourage increased participation in medical decision making and personal health choices | |
Reduced interest in family-centric activities | Supplement parental support with organized peer-support activities | Initiate discussions about eventual transition to adult providers | |
Increasing peer-identification | |||
Middle adolescence (14–16 years) | Importance of physical attractiveness, popularity and self-esteem | Direct conversations towards the adolescent with active involvement in decision-making | Direct the conversation towards the adolescent with active involvement in decision-making |
New understanding of abstract concepts and consequences | Provide support for body image issues, self esteem | Reserve 1-on-1 time with teen for a portion of each clinic visit | |
Reorientation of primary relationships from family to peer groups | Provide support to reduce social isolation and depression through adolescent support groups and teen-friendly facilities | Discuss prevention of high risk behaviors (smoking, alcohol, drug use, unprotected sex) | |
Start of dating | Stress importance of adherence to therapy | Discuss targets for transition readiness and provide rationale for transition to adult focused providers | |
Late adolescence (17–20 years and beyond) | Development of personal independence, core values, ethical principles and philosophy of life | When feasible, offer flexibility in treatment schedule to accommodate important social events, e.g., graduation | Encourage a primary role for older adolescent during clinic visits |
Attainment of emotional independence | Offer internet access in hospital rooms for social networking | Provide information related to reproductive health and sexuality | |
Development of intimate relationships | If desired by patient, include significant other during clinic visits | Continue education about importance and rationale for life long follow-up | |
Emerging importance of career decisions as related to self-concept and emerging societal role | Help parents realize the need for adolescent’s privacy and developing autonomy | Encourage pursuit of higher education and provide information on survivor focused scholarships and resources | |
Preparation for occupation | Stress importance of adherence to therapy | Emphasize importance of preparing for employment with insurance benefits to cover continued follow-up care | |
Help them understand insurance options available for cancer survivors | |||
Assess transition readiness (see Fig. 1.2) and coordinate transition to adult setting | |||
In contrast, during transitions associated with survivorship, especially during late long-term follow-up, the focus switches to assisting patients and families with understanding and coming to terms with the persisting health problems and/or future risks resulting from cancer treatment. In both transitions, explanations should become more detailed, commensurate with the patient’s and the family’s cognitive capacity and degree of involvement in medical decision-making. In pediatric oncology, clinicians are faced with the interesting challenge of caring for older long-term survivors who were treated as young children, yet never developed insight into and understanding of their cancer and its treatment. They return year after year with little knowledge as to why they are in the cancer clinic. As these survivors mature into adolescence, it is essential they receive sufficient information about their cancer, its treatment, and the resulting health implications, in order to prepare them properly for health care transition.
1.3 The Transition from Completion of Cancer Treatment to Initial Follow-Up
This period encompasses the end of treatment until approximately 24 months after completion of treatment, during which most survivors are at the highest risk for relapse. The primary focus during this transition is to assist the patient in returning to baseline function. Ironically, toward the latter phases of treatment, many older patients develop a certain comfort level in receiving chemotherapy, especially if it is tolerated reasonably well and no relapse has occurred. The transition to end of therapy may elicit anxiety and fears related to relapse, for which many families feel unprepared [4].
One way to aid families in navigating this transition is to have a formal conference with them at the end of treatment [5]. This conference should involve at least the patient, parents and/ or significant others, the primary oncologist, and ideally the primary nurse and social worker. During this end of treatment conference, the team should briefly summarize the cancer diagnosis, the treatment received, immediate plan for follow-up, surveillance and other health recommendations. Many families are relieved to discover that they are not now “on their own,” but that cancer treatment is followed by a formal phase of surveillance with systematic monitoring initially for relapse, and then later for long-term health and well-being. Straightforward conversation about the risk and typical patterns, the timing and symptoms of relapse, when to call the oncology clinic and the specific plan for surveillance will help reduce anxiety. Parents and patients should be briefly re-educated about the relevant, major long-term effects of treatment and need for continued follow-up. This presents an excellent opportunity to introduce the concept of lifelong survivorship care and the long-term follow-up program, if such a program exists in the institution. Parents should be encouraged to re-establish their child’s care with the child’s primary care provider, whom they should now contact for any health issues except those clearly related to the cancer. Specifying when to resume childhood immunizations and normal activity should be discussed. A copy of the treatment summary and follow-up plans should be provided to the older patient, parents and the primary care provider as a roadmap or guideline for future healthcare. Names and updated contact information for organizations providing further information and support to survivors may be provided.
1.4 The Transition from Initial to Long-Term Follow-Up Care
The transition period begins approximately 2 years post-cessation of cancer treatment and continues onwards. This transition is, for most patients, open-ended in the sense that life-long surveillance is recommended for most childhood cancer survivors. The separation between initial and subsequent periods of follow-up care is not uniformly distinct, as the risk for late relapse differs by cancer diagnosis. Indeed, for institutions where referral to cancer survivorship programs occurs relatively early, long-term follow-up services can overlap and should continue parallel with disease-directed surveillance for some period of time. The primary focus of this transition is to establish the practices for risk-based monitoring and to provide related health education to the survivor and family. Whereas the major risk during the initial period of follow-up is relapse, the major risk during this later period is disengagement from medical care and failure to remain in structured follow-up.
1.4.1 Late Effects and the Need for Survivorship Care
While the incidence of childhood cancer has increased gradually over the past three decades, mortality due to childhood cancer has steadily decreased [6]. In 2005, an estimated 328, 652 childhood cancer survivors were alive in the United States [7]. The prevalence of childhood cancer survivors has been estimated to be approximately 1 in 640 among Americans aged 20–39 years [8]. These figures will undoubtedly increase in the future as survival continues to improve.
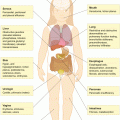
Many survivors remain at increased, life-long risk for clinically significant complications of their cancer therapy. These are commonly referred to as “late effects,” defined as any chronic or late-occurring outcome, physical or psychosocial, that persists or develops 5 years after the cancer diagnosis [8]. In an analysis of self-reported data from 10,397 survivors and 3,034 siblings, investigators from the Childhood Cancer Survivor Study (CCSS) found that the risk for a chronic or life-threatening health problem was 3.3 and 8.2 times higher, respectively, in survivors compared with their siblings. The cumulative incidence of one or more chronic health conditions reached 73.4 % 30 years after the cancer diagnosis, with a cumulative incidence of 42.4 % for conditions graded as severe, disabling, or life-threatening [9]. A study involving 1,315 survivors from the Netherlands reported similar findings [10]. The excess risk does not appear to reach a plateau with time [9, 10]. Data from the CCSS indicate that all-cause mortality is 8.4 times higher among survivors compared with the United States (US) population 25 years following cancer diagnosis [11]. Although recurrent/progressive disease accounted for most deaths, second or subsequent cancers and cardio-pulmonary late effects were noted to become important contributors over time [11]. Selected late effects by organ system, their risk factors and recommended surveillance tests are outlined in Table 1.2.
Table 1.2
Overview of selected late effects in childhood cancer survivors
Organ system | Late effect | Risk factors | Surveillance |
|---|---|---|---|
Neurologic | Neurocognitive delay | Methotrexate, cytarabine, RT | Neuropsychological testing |
Leukoencephalopathy | Methotrexate, cytarabine, RT | Neurologic examination, MRI | |
Peripheral neuropathy | Vincristine, vinblastine | Neurologic examination | |
Endocrine | Hypothyroidism | RT | TSH, free T4 |
Growth hormone deficiency | RT | Growth chart | |
Gonadal failure | RT, alkylators | Testosterone, estradiol, FSH, LH | |
Cardiovascular | Cardiomyopathy | Anthracyclines, RT | Serial echocardiography |
Coronary artery disease | RT | Clinical history | |
Carotid artery narrowing | RT | Carotid artery ultrasound | |
Pulmonary | Pulmonary fibrosis, restrictive or obstructive lung disease | Bleomycin, busulphan, lomustine, carmustine, RT | Chest X-ray, pulmonary function testing |
Genitourinary | Reduced GFR | Cisplatin, RT | Serum creatinine |
Tubular dysfunction | Cisplatin, ifosfamide | Serum electrolytes, Mg, Phos | |
Hemorrhagic cystitis, bladder fibrosis | Cyclophosphamide, ifosfamide, RT | Urinalysis | |
Reproductive | Infertility | Alkylators, RT | Clinical history, specialty assessment |
Gastrointestinal | Cirrhosis | RT | Liver function test |
Chronic enterocolitis | RT | Clinical history | |
Strictures | Surgery | Clinical history | |
Musculoskeletal | Osteopenia/osteoporosis | Corticosteroids, methotrexate | Bone density measurement |
Osteonecrosis (AVN) | Corticosteroids | Clinical examination, MRI | |
Altered bone growth | RT | Clinical examination | |
Eyes | Cataract | Corticosteroids, RT | Regular eye examination |
Auditory | Hearing loss, tinnitus | Cisplatin, RT | Audiological evaluation |
Oral | Dental caries, dry mouth, dental maldevelopment | RT | Regular dental examination |
Psychosocial | Post-traumatic stress syndrome, interpersonal difficulties, special educational needs, career and vocational challenges, insurance deficits | The cancer experience; functional disabilities arising from specific late effects | Clinical history, psychological evaluation, social work assessment |
Secondary neoplasms | Melanoma, breast carcinoma, thyroid carcinoma, sarcoma, bowel cancer, brain tumor | RT | Site specific surveillance |
Acute myeloid leukemia/myelodysplastic syndrome | Etoposide, anthracyclines, RT
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|