The same lack of technical improvements applies to various different steps along the histopathological processing. Tissue fixation is still conducted manually and with the use of formaldehyde originally proposed by Blum [2, 14]. Moreover, grossing and decalcification are still carried out by manual, traditional processes. Not only, lack of technical improvements is paralleled by lack of proper standardization, so that concentration of the reagents time and temperature of use vary in different laboratories worldwide according to local habits.
This status of affairs is perhaps understandable, since until recently the sole criterium of validity of histological preparations was morphological preservation and structural patterns, comprehensive of ultrastructural images, were the main basis for disease classification.
In more recent times, the use of histochemical and immunohistochemical techniques has allowed pathologists to develop more precise, reliable, and reproducible disease classifications and to complement morphology with information regarding protein (antigen) expression and distribution. It has thus become mandatory to rely on technical preparation providing sections optimal for microscopical observation, but at the same time preserving the biological integrity, particularly of the proteins and nucleic acids.
Since year 2000, pathologists have made critical steps forward in the knowledge of the pathogenesis and genetic profiles of several cancer types and this has made significant impact on prospects for both cancer prevention and the use of novel personalized therapeutic regimens.
Lately, attention has moved toward gene analysis as a method of examining both origin and differentiation of various tumor types. Molecular analysis is thus emerging as an essential technique to complement conventional histopathology. This is reflected by the progressive evolution of the WHO Classification report outlined in the “Blue Books.” This report initially only dealt with histological features, but more recent editions use the results of genetic analysis to complement, but never substitute for, the morphological characterization [3, 13, 39].
The consequence of this improvement is that cancer diagnosis for individual patients has become more complex and molecular tests have become mandatory, for example, for the identification of gene mutations responsible for familiar hereditary tumors of endocrine organs (MEN 1 and MEN 2 Syndromes) [23, 45] or the assessment of microsatellite instability for the identification of carriers with increased risk for Lynch Syndrome [32, 35]. Nowadays, morphological diagnoses are not sufficient for planning personalized therapies that need, for example, the detection of chromosomal translocations and aberrations in sarcomas and brain tumors [4, 21, 22, 34, 44] the evaluation of mutations of EGFR and K-RAS genes in lung and colorectal adenocarcinomas [25, 33, 37, 38], of cKIT and PDGFRA genes in cases of gastrointestinal stromal tumors (GISTs) [1, 19, 26] and of BRAF and NRAS genes in melanomas [17, 41], and last but not least the evaluation of the status of the HER2 for breast cancer [10, 28].
These tests require a proper preservation of tissues, to be analyzed in parallel for tissue structural arrangement, protein (antigen) distribution, and gene sequencing. Variations on the conditions of fixation and paraffin embedding heavily influence both structural and chemical preservation, thus bearing great impact on diagnostic definition and, ultimately on therapeutic treatment.
We are in conclusion witnessing a severe gap between the request for proper, selected, and standardized processing of pathological tissues and lack of novelty in the offer by the industry of dedicated apparatuses. This gap is, in our opinion, critical in the areas of (1) Tissue transfer, (2) Grossing, (3) Fixation, (4) Decalcification, and (5) Fast Embedding in Paraffin.
2 Tissue transfer
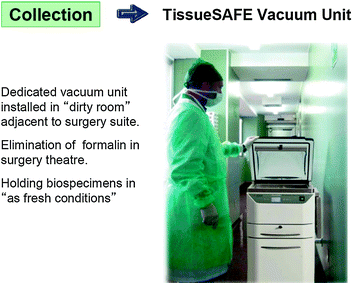
Once surgically removed from the body, the specimen has to be transferred to the pathology lab for histopathological examination. Time and conditions of transfer are critical, since fresh tissues are susceptible to autolytic process, which are bound to affect structure and biochemical components. Ways of transfer of surgical specimens vary according to the architectural layout and distance between surgical theaters and pathology laboratories. The ideal situation is when physical location and hospital protocols allow for the immediate transfer of “fresh” tissue specimens for prompt grossing and fixation. Accordingly, it has been recommended the “ischemia time” to remain below 1 h to achieve a proper processing of breast cancer specimens, permitting a correct evaluation of both morphological and therapeutic-prognostic parameters [15, 43]. Problems arise when this is not feasible being the pathology laboratory located far away from the surgical theater. It is a common practice in many hospitals, to immerse large specimens and organs into large formalin-filled containers, which are then transferred to the pathology laboratory usually once every day. This practice carries problems, since autolysis will continue in central areas of the specimen. Protection of fresh tissues from drying is essential, and this can be achieved by sealing tissues under-vacuum in a plastic bag [6]. This offers the additional advantage of removing air, thus oxygen with the double advantage of blocking aerobiotic processes and of facilitating cooling of specimens because of the lack of insulating air. Our company has been patenting an dedicated apparatus (Tissue Safe, patent EP 2070410 B1) which meets these demands and encountered the approval of several laboratories.

3 Grossing
Histopathological processing of specimens larger than 2 cm requires grossing and selection of sections from significant areas, a process which can only be performed in pathology laboratories by qualified personnel In order to favor penetration of reagents, into the tissue, sections that are to be processed in “bio-cassettes” should not be thicker than 3–4 mm.
Still, the grossing of fresh and soft tissues is not easy. Our company, in order to facilitate the grossing at proper thickness has been devising and producing special forceps (CUT-Mate forceps).
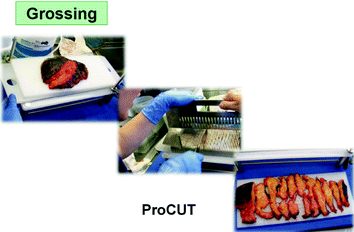
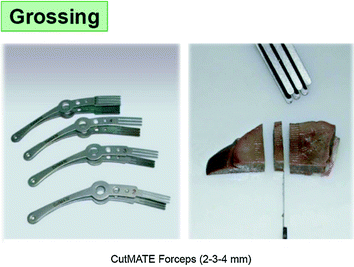
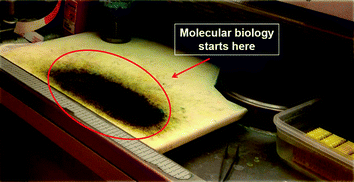
The grossing time is at risk of incorrectly mixing specimens from different cases (and patients). Until recently, the consequences of this risk were ultimately minor and did not influence the final diagnosis, since minute residues of “external” source could be histologically recognized and therefore dismissed. The advent of molecular analyses applied to tissue blocks has changed the situation, because of the lack of histological control and of the sensitivity of the procedures. It has therefore become essential to avoid the risk of mixing of specimens, as could be for instance due to the use of a single table for the grossing of all specimens. Our company has developed special polypropylene sheets to be placed and held on top surface of the cutting board. A new one for each single specimen. These sheets provide a safe, simple, and effective solution of the problem of contamination of specimens. In addition, the sheets can be washed in the cab washer during the night to be reused the next day.

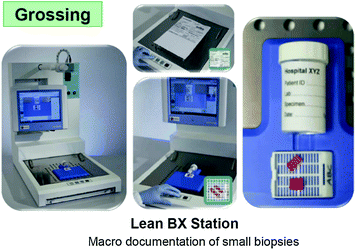
Once grossed, the surgical specimens are not recognizable any more, while documentation of the original specimen, of the site of the grossing and a measure of the different areas is essential both, for the pathologists when reading the slides and for a proper and documented discussion with surgeons, clinicians, and radiologists. Our company has devised the Macro-Path apparatus which meets these requirements, by digitally documenting the whole grossing process, including sizing, sections, and area of disease.
4 Fixation
Fixation is the process whereby cell and tissue structures, as well as chemical components are preserved in their integrity. This process is most commonly accomplished by immersion into a fluid, which gradually penetrates and acts chemically and/or physically.
A 4 % formaldehyde solution in water (formalin) has been adopted as the fixative of choice in histopathology as it is relatively cheap, easy to use. It is also reliable because it does not overfix and it guarantees, in appropriate conditions, an optimal morphological preservation so that we have to conclude that substitution of formalin with alternative fixatives cannot be foreseen at present [8].
However, length, temperature, and modalities of formalin fixation are not standardized and vary extensively in different laboratories.

The length of formalin fixation can affect the results of the immunostaining [9, 18, 27] since under fixation often produces a reduced immunostaining in the central region of the tissue block with stronger immunoreaction in the marginal area of the section, while overfixation generates the opposite aspect (good staining in the inner area and poor staining outside.
Moreover, formaldehyde fixation modifies the conformation of macromolecules, altering tertiary and quaternary organizations of proteins, whereas the primary and secondary structures are scantily affected [12, 29, 31]
Such conformational changes may hamper the link to the antibody, but the use of antigen retrieval procedure can return immunoreactivity in formaldehyde-fixed specimens [40].
Finally, if formalin does not penetrate completely the tissue during the fixation process, the subsequent use of ethanol (as a dehydrating agent) during paraffin embedding may produce in the tissue blocks central areas which are predominantly alcohol-fixed, and this can procedure additional variable results [20, 42].
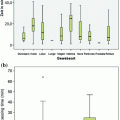
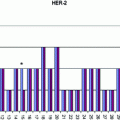
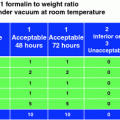
This issue is particularly relevant in oncopathology for the evaluation of factors predicting responsiveness to therapeutic treatments, and thus, fixation in phosphate buffered formalin (PBF) of breast cancer tissue blocks for no less than 6 and no more than 48 h is now required in order to guarantee an optimal evaluation of Estrogen (ER) and Progesterone Receptors (PgR) and HER2 expression by immunohistochemistry [15, 43].
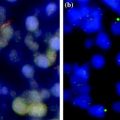
In more recent times, a crucial request in cancer pathology has been nucleic acid preservation for gene expression profiling, with the goal of generating new and reliable diagnostic and prognostic parameters. Studies conducted on the preservation status of nucleic acids in FFPE tissues generally agree on the relatively good (though not optimal) preservation of DNA. On the contrary, RNA has been found to be heavily degraded and fragmented so that only short sequences, approximately 100–200 nucleotides long, can be recognized and amplified [11, 30, 36].
Innovative protocols have, however, been proposed or permitting gene expression profiling on FFPE tissues from cancer patients [7].
A variation in formalin fixation resulting in improved preservation of RNA has recently been proposed by Bussolati et al. [5].
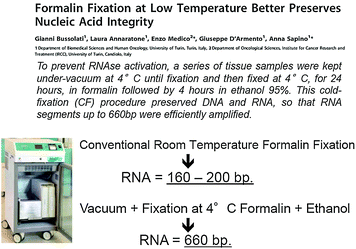
This is based on a fixation process in formalin at 4 °C, followed by dehydration in cold ethanol and paraffin embedding (CFFPE). Using this procedure, we succeeded in obtaining a substantial reduction in RNA fragmentation in FFPE tissue blocks, as assessed by RT-PCR and gene array analysis, while at the same time preserving the morphological and immunohistochemical properties which make formalin the fixative of choice in histopathology.
5 Decalcification
Decalcification is the process whereby mineralized tissues are currently examined histologically on sections from paraffin-embedded blocks. Removal of calcium salts is effected, on fixed tissues, by agents (acids or chelating agents) leaving unaltered the protein components and the structure of the tissue. This process, in histopathology, is currently used for the study of bone tumors and of bone marrow biopsies, a diagnostic procedure of great value in hematopathology. Decalcification is carried out almost universally in a manual manner, without any standardization of time–temperature, pH, agitation, and reagents management.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree