Fig. 22.1
Endoplasmatic reticulum and gene expression
22.3 Epidemiological Data That Demonstrate the Safety of Isoflavones
Alongside a large number of experimental facts that suggest the chemoprotective effect of isoflavones, there is also a wealth of epidemiological data demonstrating with a high level of medical evidence the protective effects of isoflavones vis-à-vis hormone-dependent malignancies:
A publication by the European Prospective Investigation Group appeared in Breast Cancer Research and Treatment in April 2013. In this work, at least 334,000 women between the ages of 35 and 70 years were evaluated in 10 European countries. The work showed that there is no risk whatsoever associated or linked with isoflavones as far as breast cancer is concerned [1].
Another study (Boucher et al.) was published in the IJC (International Journal of Cancer). There, around 3000 cases were compared with 3000 controls; in this study, yet another aspect emerged as well. Isoflavones possess a protective effect with regard to the development of breast cancer [2].
As early as 2007, a working group in the Netherlands (Verheus et al. 2007) published prospectively collected data in the Journal of Clinical Oncology. A group of 200 women showed that the measurable level of isoflavone in the blood correlated directly with protection from breast cancer. The higher the serum level of isoflavone, the lower the likelihood of developing breast cancer. The study was carried out on the basis of a scrupulous methodology [3].
Also published in Journal of Clinical Oncology was a publication from Israel and Hong Kong that included 24,000 women and substantiated a familiar finding: high levels of isoflavones in the blood are associated with a markedly low incidence of breast cancer [4].
A work by Magee, P.J., and Rowland, I., that appeared in January 2013 posed the provocative question: is it legitimate to use soya following a malignancy? The authors answer the questions with: Yes – it is legitimate, and there is no interference with tamoxifen or with an aromatase inhibitor such as anastrozole [5].
In the ‘Shanghai Breast Cancer Survival Study’, Prof. Shu of Vanderbilt University (USA) observed 5043 women for 5 years following breast cancer therapy and who were given a daily dosage between 30 mg and 70 mg of isoflavones and 11 g of soya proteins. Clinical data demonstrated a significant improvement in the 5-year survival rate, a significantly lower rate of recurrence and a synergism in combination with tamoxifen [6].
A follow-up study tracked 524 breast cancer patients who had undergone surgical treatment. All were treated with adjuvant anastrozole, and 85 % of the patients were ER+ and PR+. The average follow-up period was 5.1 years. A statistically significant reduction in the risk of recurrence was observed in the most vulnerable subgroup of postmenopausal women [7].
DKFZ has also published a work online that found that among postmenopausal patients with breast cancer, not only the mortality rate but also the risk of developing metastases or secondary tumours were up to 40 % lower among patients with diets rich in soya [8].
Even in the past, it was demonstrated several times over that components of soya and red clover had no negative effect on hormone-dependent malignancies. The two publications by the groups from the Netherlands and Israel, however, go a step further and corroborate the suspicion that, on the contrary, there is a high likelihood that isoflavones have a protective effect on the development of hormone-dependent malignancies.
Still, some time ago, professional societies such as the Austrian Society for Reproductive Medicine and Endocrinology already stated their positions on the matter [9]. They made it clear in no uncertain terms that the breast-cancer-preventive effects of isoflavones had been demonstrated with a relatively high level of evidence.
This is not just limited to breast cancer, however. As a meta-analysis (KORMA) Study Group [10] showed, this finding applies to hormone-dependent ovarian and endometrial cancers in the gynaecological area as well; the protective effects of isoflavones have been presented here too.
22.4 The Complexity of Evolution
Evolution knew how to enlist the same substances for different tasks, thus significantly expanding its reach. Endocrinology is no exception to this principle. For instance, a hormone can be converted into various other hormones; the best known example is testosterone, which is converted to oestradiol through the workings of aromatase. On the other hand, with a variety of receptors available for a single hormone, this also enables physiological diversification. There are two receptors for oestrogen in higher mammals: oestrogen receptor alpha (ER-α) and oestrogen receptor beta (ER-ß). Their influence is similar to that of a yin and yang system.
22.4.1 The two Oestrogen Receptors Can Be Selectively Activated
The oestrogen receptor familiar to researchers for longer is known as ‘ER-alpha’. In 1995, J.-Ă. Gustafsson discovered another oestrogen receptor, the ER-beta (ER-ß). Both are localised within the nucleus. Such nuclear receptors can be activated by low-molecular-weight substances such as oestrogens and structurally similar molecules, e.g. isoflavones, or by synthetic active ingredients. A precondition to activation of the receptors is that these substances pass through the membranes of their target cells, continuing through the cytoplasm of the cells to the nuclei, where they bond covalently with the receptors in the nuclei. They then form a ligand-receptor complex. Once activated by a ligand, ER-α causes ‘cellular activity in the broadest sense’, including proliferation and inflammation; this can result in an increased risk of mutations and the oncogenesis to which this can lead.
ER-ß, on the other hand, once activated by a ligand, provides for ‘calming’, for anti-proliferative and anti-inflammatory effects and hence for an option to protect against cancer through targeted activation of this receptor using suitable substances. This dimension also gives rise to treatment possibilities of the kind already seen with the oestrogen receptor-beta agonist tamoxifen.
22.4.2 Does ER-ß Provide Protection from Hormone-Dependent Cancers?
When it encounters ER-alpha, 17-beta oestradiol (E2) can stimulate mitosis and promote inflammation and angiogenesis. If, however, ER-beta predominates in the tissues, then the same stimulant, oestrogen, inhibits mitosis and has anti-inflammatory and antiangiogenic effects. Thus, the same substance can have two different effects, depending on the receptor constellation in the tissues.
The best evidence for the chemopreventive effect of the oestrogen receptor beta is provided by tamoxifen, referred to above, which prevents the tissue against further proliferation via the selective effect on the beta receptor in the breast.
Soya isoflavones are natural ER-ß agonists. They selectively stimulate ER-ß and promote ER-ß expression in tissues as well.
22.4.3 Isoflavones Intervene in Paracrinology
There are not only the different receptors ER-ß and ER-α, but there are also different metabolites of E2. Today, we know that the oestradiol, progesterone and testosterone are only precursors, i.e. precursor steroids. From these precursors, each organ can then assemble the hormone it needs in its own local situation.
The metabolism of oestradiol was presented for the first time at the major cancer congress (Conference of the National Cancer Institute) in Bethesda, Maryland, in 1998. It is evident that the original oestradiol can be hydroxylated at a different place in the molecule; this diversifies the effectiveness of the hormone in different directions.
The human body accomplishes this with other hormones in different organs as well. This has long been known and accepted for testosterone. In the prostate, it is hydroxylated further into dihydrotestosterone, which ultimately exerts the stimulative effect on the prostate gland. Progesterone, too, is transformed into other metabolites in the brain, such as allopregnanolone, which also has a highly differentiated task to perform. The situation with oestrogen is similar. It has three different highways available to it that it pursues following release from the ovary or following oral administration (Fig. 22.2).
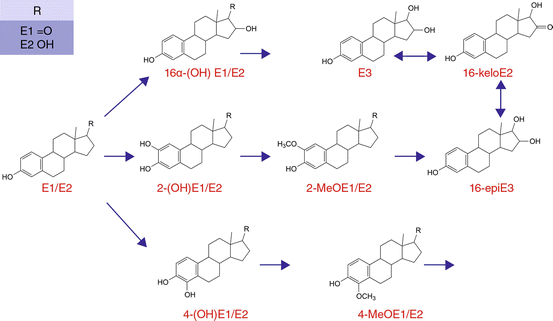





Fig. 22.2
Oestradiol metabolism
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree