Fig. 27.1
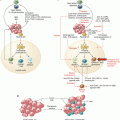
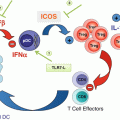
The immunogenicity of E. hirae. E. hirae resides in the jejunum and colon in about 25% individuals. It is very dominant in mouse guts at Gustave Roussy Villejuif. This Enterococcus is not pathogenic and benefits from cyclophosphamide-mediated increased gut permeability to translocate in secondary lymphoid organs, prime specific pathogenic CXCR3 + CCR3+ T cells that traffic to tumor lesions, and reprogram the tumor microenvironment, i.e., decreasing regulatory T cells and γδT17 cells and enhancing cancer-specific CTL responses. E. hirae also acts on the intestinal epithelial cells, modulating apoptosis and IL-18 secretion in a NOD2-dependent manner. E. hirae is recognized by bone marrow dendritic cells to induce IL-12 and IL-27 production in a NOD2-dependent fashion. All these properties participate to its adjuvant effects to boost cyclophosphamide-mediated immunostimulatory capacities and anticancer efficacy
Therefore, part of the immunomodulatory effects of the most well-known immunomodulator (CTX) require a functional microbiome, at least a Gram-positive small intestine residing Enterococcus and a colon residing Barnesiella.
27.6 Platinum Salts and Gut Microbiota
The demonstration of the importance of the gut microbiota on the efficacy of anticancer agents was also reported by the Dr. Giorgio Trinchieri’s group. Iida et al. demonstrated that bacteria accounted for the ROS-mediated antitumor effects of tumor-infiltrating myeloid cells during platinum-based anticancer therapies [61]. Indeed, the authors showed that the efficacy of platinum salts (oxaliplatin, cisplatin) relied on the production of reactive oxygen species (ROS) by intratumoral myeloid cells (myeloid suppressor cells, monocytic and immature dendritic cells) through a mechanism involving MyD88. ROS induce DNA damage culminating in DNA adduct formation and apoptosis of tumor cells and account for part of the tumoricidal activity of oxaliplatin against MC38 or EL4 tumor-bearing mice. Interestingly, the efficacy of chemotherapy was abrogated in germ-free or ATB-treated mice compared to SPF animals. Next, the authors investigated the impact of ATB treatment on oxaliplatin-mediated ROS production. The expression of Cybb encoding reactive oxygen species (ROS)-generating nicotinamide adenine dinucleotide phosphate oxidase (NOX2) was attenuated by the ATBs [61]. Furthermore, microbial compensation of ATB-treated mice with Lactobacillus acidophilus restored efficacy of cisplatin-based chemotherapy and reestablished inflammatory gene expression related to cisplatin functions [62]. Altogether, these results support the notion that the gut microbiota impacts on the redox equilibrium of the tumor microenvironment, affecting the therapeutic effects of platinum salts currently used in clinic.
27.7 Gut Bacteria and the Efficacy of Immuno-oncological Compounds
Oncologists no longer consider to treat cancer by only targeting tumor cell clones but also by mobilizing the immune system and eliciting long-term memory T cell responses protecting against the minimal residual disease. Immune checkpoint blockade (ICB) became the backbone of cancer treatment modalities, but primary resistance to ICB concerns about 70% of all comers, suggesting that T cell responses culminating in T cell infiltration of tumor lesions required for tumoricidal effects are quite a rare event. The immunity of a person is influenced by a complex set of factors, i.e., host, tumor, and environmental cues as well as prior histories of distinct therapies, that govern the threshold and timing of anticancer immune responses. Several lines of evidence point out to the critical role of the microbiome in dictating the “cancer immune set point” of a person, i.e., the threshold beyond which an immune response will ensure. Several groups indeed highlighted the role of distinct commensals in determining the antitumoral efficacy of various types of immunotherapeutics.
After administration of anti-interleukin-10 receptor (IL-10R) plus cytosine-phosphate-guanosine oligodeoxynucleotides (CpG-ODN) TLR9 agonists, Alistipes shahii was found overrepresented in the feces of colon cancer-bearing mice. Upon mono-association of previously sterilized mice with A. shahii, the immunotherapeutic response of subcutaneous colon cancers was improved, as compared to sterile mice. In this model, A. shahii led to an increase of TNFα production by intratumoral myeloid cells, and neutralization of TNFα abolished the therapeutic effect. Thus, A. shahii impact innate immune effectors of the myeloid lineage, reshaping the tumor microenvironment to improve the outcome of immunotherapy [61].
Other studies showed that the efficacy of immune checkpoint blockers (ICB), aimed at reactivating tumor-infiltrating T cells, is also dependent on the gut microbiota. Ipilimumab, a monoclonal antibody against cytotoxic-T-lymphocyte-associated protein 4 (CTL4-4), induced considerable improvement in the overall survival of patients with metastatic melanoma with up to 18% long-term control of the disease [63, 64]. The antitumoral efficacy of ipilimumab was abrogated in ATB-treated SPF mice or in germ-free animals bearing many types of transplantable tumors. Vétizou et al. showed that non-enterotoxin-producing strains of B. fragilis have anticancer properties in the context of immunotherapy with CTLA4 blockade. After neutralization of this immune checkpoint with specific monoclonal antibodies, Bacteroidales representation in the ileums and feces was affected, and B. fragilis spp. could take over, associated with DC migration and activation in the mesenteric lymph nodes and class II-restricted T cell memory responses against B. fragilis antigens. B. fragilis-mediated immune responses post-CTLA4 blockade were IL-12 dependent. The defect in preclinical response of GF tumor-bearing mice to anti-CTLA4 Ab was overcome by mono-association with B. fragilis as well as by adoptive transfer of CD4+ T cells that were previously primed with B. fragilis-pulsed dendritic cells (DCs) that contributed to the antitumor immune rejection. Interestingly, mice that were mono-associated with B. fragilis exhibited a more mature DC phenotype in tumor beds than controls with respect to the expression of MHC class II as well as the co-stimulatory CD80 and CD86 molecules. It has been documented that B. fragilis cell walls contain the immunostimulatory polysaccharide A (PSA) that can act on DCs. However, it remains to be determined whether PSA alone would be as efficient as live B. fragilis with respect to its antineoplastic activity. Finally, we were able to confirm that these findings were of clinical relevance by analyzing the gut microbiota of metastatic melanoma patients before and after ipilimumab. The 16S pyrosequencing analyses of feces contents in 25 stage IV melanoma patients revealed three major enterotypes based on the abundance or relative representativity of distinct spp. of Bacteroides and Prevotella genera. By performing fecal microbial transplantation (FMT) of feces representative of each enterotype into tumor-bearing GF mice subsequently treated with anti-CTLA4 Ab, we demonstrated that the microbial composition of enterotype C, enriched in immunogenic Bacteroides species, was able to allow the niching or colonization of B. fragilis (but not Bacteroides uniformis, vulgatus, or distasonis) and to restore the efficacy of anti-CTLA4 Ab, otherwise lost in GF mice, while clusters A and B failed to do both [65]. Of note, melanoma patients tended to exhibit an enterotype C while being treated with ipilimumab.
In parallel, Gajewski’s group showed that the antitumor efficacy of anti-PD-L1 Ab was influenced by the colon content in Bifidobacterium species (Bifidobacterium breve and Bifidobacterium longum). In this study, Sivan and colleagues compared relative antitumor CTL responses against a candidate tumor antigen in genetically similar C57BL/6 tumor bearers derived from two different mouse facilities (bought from two distinct vendors) differing in terms of microbiome composition. Contrasting mice from the Jackson Laboratory and from Taconic Farms, they revealed significant differences in the growth kinetics of subcutaneously implanted melanomas, with more aggressive tumors in Taconic Farms derived- mice attributable to lower dendritic cell maturation and IFN signatures associated with poor intratumoral cancer antigen-specific TIL accumulation. Interestingly, the tumor growth in Taconic Farms mice was reduced following FMT from mice originating from the Jackson Laboratory or cohoused with these littermates. Pyrosequencing of gene amplicons of Jackson and Taconic mice feces revealed a high content in Bifidobacteriales spp. in the colony that exhibited reduced growth of transplantable melanomas and improved CTL-mediated immunosurveillance [4]. Selective transfer of B. breve or B. longum into mice that are normally devoid of these strains was sufficient to reduce melanoma growth and restored anti-melanoma CTL responses. B. breve and B. longum stimulated the maturation of DC both in vitro and in vivo. As a consequence, the frequency of tumor-specific CTL accumulating in melanomas increased in mice carrying B. breve or B. longum, and such CTL-infiltrated tumors responded more vigorously to immunotherapy with an antibody targeting PD-L1 than did melanomas evolving on sterile mice or mice bearing a gut microbiome devoid of immunostimulatory Bifidobacteriales [66].
Hence, various strains of commensals were associated with the immunostimulatory effects of distinct I-O strategies. The future will tell us whether most of these commensals would mediate their bioactivity on innate or cognate immune responses regardless of the therapeutic compound or whether they would act within the mode of action and scope of the defined compound.
27.8 Role of Intestinal Microbes in Graft-Versus-Host Disease (GVHD)
Graft-versus-host disease occurs after allogeneic hematopoietic stem cell transplantation (allo-HSCT) and often limits the success of the therapy. GVHD results from the attack of the host cells by the transplanted donor immune cells. Numerous studies demonstrated that GVHD depends on several factors such as age, conditioning regimen, graft source, etc. Moreover, increasing evidences indicate that the gut microbiota plays a significant role in the pathogenesis of GVHD and could be associated to the relapse of hematologic malignancies after allo-HSCT. Previous studies performed in mice showed that the severity of GVHD is attenuated in GF mice or mice treated with antibiotics [67, 68] or in humans [69]. Characterization of the gut microbiota of GVHD patients revealed a significant decrease of diversity which was associated with an increase of Lactobacillales and decrease of Clostridiales, which in turn can modulate intestinal inflammation [70]. In a later study, Jenq et al. anticorrelated the abundance of Blautia, belonging to the Clostridia class, with GVDH-related mortality [71]. While bacterial species known for their health-promoting properties such as Faecalibacterium or Ruminococcus were deeply decreased, the abundance of Enterococci was markedly enhanced in GVHD patients, compared with patients without GVHD [72]. Indeed, an increase of Enterococci after transplantation in adult patients with severe GVHD was noticed. This was also observed in ten pediatric patients undergoing allo-HSCT. Interestingly, patients undergoing GVHD displayed lower amounts of Bacteroides and Parabacteroides, whose abundance positively correlated with the levels of SCFAs, especially propionate, before allo-HSCT [73]. Lately, Simms-Waldrip et al. demonstrated that the use of antibiotics targeting anaerobic bacteria in the course of allo-HSCT correlated with a significant decline of anti-inflammatory Clostridia and the development of GVHD [74]. As a result, manipulating the gut microbiota through the use of probiotics or prebiotics could be considered as a therapeutic approach aiming at decreasing the risk of GVHD. Preliminary data indicated that administration of Lactobacillus rhamnosus before and after allo-HSCT promoted less severe GVHD and improved survival of mice [75]. Other studies observed that administration of Lactobacillus johnsonii reduced GVHD severity through prevention of Enterococci accumulation post allo-HSCT [76]. Moreover, FMT was used in a clinical pilot study to treat GVHD of the gut occurring after allo-HSCT [77]. The abundance of Lactobacilli increased post-FMT in most of the patients, and the gut microbiota shifted toward an anti-inflammatory state. Other studies showed that FMT was efficient in treating Clostridium difficile infection post allo-HSCT [78, 79]. Butyrate-producing Clostridia strains reduced GVHD of the gut that is induced by allogeneic BMT. This effect was correlated with improved junctional integrity and reduced apoptosis of intestinal epithelial cells [80].
In a recent study, Peled et al. reported that intestinal abundance of Eubacterium limosum was associated with a reduced risk of relapse/progression of hematologic malignancies after allo-HSCT. Interestingly, the strongest association between E. limosum and a lower risk of disease relapse was observed in patients receiving T cell replete grafts. Donor cell-mediated graft-versus-tumor (GVT) effect was virtually higher in these patients, when compared to T cell-depleted graft recipients, suggesting that gut microbiota might play a role in GVT activity [81].
Altogether, these studies highlighted the dominant role of gut microbiota in dictating the efficacy and toxicity of allo-HSCT and open up new avenues of interventions on GVHD/GVT by harnessing the gut microbiome.
27.9 Bacteria for Therapy of Cancer: Probiotics and Others
The development of anticancer agents based on live microbial agents traditionally focused on local or systemic parenteral routes. William Coley was the first who partially succeeded in obtaining anticancer effect using microbes, by injecting intratumorally a mixture of Streptococcus pyogenes and Serratia marcescens [82]. Many efforts were then made to develop microbial-based anticancer treatment. Mycobacterium bovis Bacille Calmette-Guérin (BCG) was approved by FDA and EMA in 1990 for superficial bladder cancer treatment. BCG efficacy relies on the induction of a local immune response against residual cancer cells, reducing the probability of relapse [83, 84]. Since then, no other bacteria obtained marketing authorization despite Phase I clinical trials utilizing various strategies outlined in Table 27.1.
Table 27.1
Clinical trials utilizing live bacteria
Bacterial species | Cancer | Clinical benefit | Refs |
|---|---|---|---|
Streptococcus pyogenes and Serratia marcescens | Osteosarcoma | Coley’s toxins: Injection of Streptococcus pyogenes and Serratia marcescens in sarcoma patients leading to tumor regression | [82] |
Mycobacterium bovis BCG | Superficial bladder cancers | Intravesical administration of a live attenuated form of Mycobacterium bovis leading to decreased risk of local recurrence | [97] |
L. Casei Shirota (found in the fermented milk Yakult) | Superficial bladder cancer | NK and macrophage stimulation leading to decreased tumor recurrence | |
IMM-101 (heat-killed Mycobacterium obuense; NCTC 13365) with gemcitabine | Melanoma Advanced pancreatic adenocarcinoma | Increased survival in metastatic disease in a randomized Phase II study | |
Live-attenuated Listeria monocytogenes expressing mesothelin (CRS-207) with GVAX-cyclophosphamide | Advanced pancreatic ductal adenocarcinoma | Increased overall survival associated with mesothelin-specific CD8 Tc1 responses | [103] |
IL-13-PE: Recombinant cytotoxin consisting of human interleukin-13 (IL-13) and a truncated form of Pseudomonas exotoxin A (PE) | Adrenocortical carcinoma Phase I | Feasibility and neutralizing antibody responses | [104] |
IL4-PE: Chimeric fusion protein composed of IL-4 and Pseudomonas exotoxin | Astrocytoma Phase I | No toxicity, median survival of 8.2 months and evidence of necrosis on MRI in several patients | [105] |
Attenuated strain of Salmonella typhimurium, VNP20009 | Metastatic melanoma Refractory solid tumors Phase I | Evidence of bacterial tumor colonization but objective response | |
TAPET-CD: An attenuated Salmonella bacterium expressing the E. coli cytosine deaminase gene | Three patients (one head and neck squamous cell and two adenocarcinoma of the esophagus). Intratumoral injection | Evidence of bacterial colonization and conversion of 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU) in 2/3 tumors | [108] |
Tf-CRM107 is a conjugate of transferrin and a point mutation in the diphtheria toxin | 15 patients with malignant brain tumor | MRI regression of tumor volume in 9/15 patients with no evidence of severe local and systemic complications at low dose | [109] |
Investigators recently raised an alternative approach that consists in administering live bacteria (probiotics) per os to colonize the gut and consequently obtain anticancer activities.
Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer health benefits on the host” [85]. They are known to reinforce natural defenses, protect against gastrointestinal disorders and pathogens, and modulate innate and/or adaptive immunity.
Lactobacilli are substantial probiotics, which belong to the group of lactic acid bacteria. Many studies demonstrated that various isolates of Lactobacillus casei, Lactobacillus plantarum, Lactobacillus rhamnosus GG, and/or L. acidophilus mediate anticancer effects through different mechanisms, including activation of NK cells, maturation of DCs, or release of probiotic-derived ferrichrome [86–95].
Prohep, a probiotic mixture of Lactobacillus rhamnosus GG (LGG), Escherichia coli Nissle 1917, and heat-inactivated VSL#3, delivered orally to mice bearing subcutaneous hepatocellular carcinoma, prevented tumor progression, and shifted the gut microbial community toward Prevotella and Oscillibacter. These later are known to produce anti-inflammatory metabolites, which in turn decrease the Th17 polarization and enhance the differentiation of anti-inflammatory Treg/Tr1 cells in the gut [96].
Whereas these probiotics taken individually may elicit antitumor activities, it is not established if when optimally combined they can create an ecosystem with extensive antitumor properties.
As mentioned previously, some intestinal commensals modulate the antitumor immune responses generated by anticancer compounds as well as the efficacy of the treatment (CTX and E. hirae/B. intestinihominis [60], CpG-ODN + anti-IL10 and Alistipes shahii [61], anti-PDL1 and Bifidobacterium longum and breve [66], anti-CTLA4 and B. fragilis/Burkholderia cepacia [65]).
The mechanisms of action of the microorganisms described above are not fully deciphered at the molecular level. The identification of bacterial structures directly involved in anticancer activity or immunosurveillance promotion will raise favorable circumstances to (a) improve antitumor effect via probiotic engineering or (b) to develop compounds that mimic their pharmacological activity.
27.10 Genetically Modified Bacteria
Din et al. demonstrated that synthetic engineering of bacteria improved the antitumor effects of the antimetabolite 5-FU in a mice model of liver metastases of colorectal cancers. Pulsatile delivery cycles of 5-FU by the bacteria were allowed via a synchronized lysis cycle of E. coli based on quorum sensing feedback loops [110]. In necrotic tumor characterized by low vascularization, chemotherapy has limited efficacy due to restricted accessibility. This can be partially restored using anaerobic bacteria as tumor-targeting vectors. Bacteria engineering increased 5-FU efficacy in liver metastasis, but this strategy was fully successful when combined with I-O or other anticancer drugs [110]. This unique way to deliver compounds in avascular tumors needs to be further assessed in patients resistant to traditional therapies.
27.11 Microbial Products with Cancer-Modulating Properties
Microbial agents can synthetize a wide range of molecules that affect either antitumor immunosurveillance or growth/survival of cancer cells. One distinguishes (a) toxins, (b) ligands of pattern recognition receptors (PRRs), and (c) metabolites (short-chain fatty acids, polyamines, vitamins, secondary bile products, AhR ligands). While toxins display direct cytotoxic properties, activation of PRRs stimulates immune response, and metabolites affect the host metabolism. However, certain metabolites can also behave as PRR ligands, as it was shown for N-acetylglucosamine (a sugar subunit of bacterial peptidoglycan) acting on hexokinase to activate the NLRP3 inflammasome [111] or for tryptophan derivatives acting on the aryl hydrocarbon receptor (AhR) [112]. These approaches have been detailed in a previous report [113]. We will only recapitulate the most prominent ones that can be harnessed for oncological purposes.
Bacterial toxins are usually peptides with amphipathic alpha-helices containing cationic charges that cause the lysis of non-protected bacterial membranes. Structural analogs of toxins were developed in order to kill cancer cells. LTX-315, a synthetic peptide developed for intratumoral therapy, targets mitochondria. It also triggers necrotic cell death with immunogenic properties. This means its direct tumoricidal effects are prolonged as a consequence of danger signal emission that activates specific antineoplastic immune responses [114].
Several PRR ligands are FDA and EMA approved. Monophosphoryl lipid A (MPL), a derivative of Salmonella minnesota LPS, is used as an adjuvant in a peptide-based vaccine for the prevention of cervical carcinoma-associated strains of human papillomavirus [115]. Imiquimod, a synthetic agonist of TLR7, is topically delivered for the treatment of actinic keratosis.
Short-chain fatty acids (SCFAs) such as acetate, butyrate, and propionate are synthetized from dietary fibers and polysaccharides by the clostridial clusters IV and XIVa of Firmicutes. While acetate supports the expansion of various human cancer types [116–118], propionate and butyrate may have anticancer properties through distinct mechanisms. Propionate and butyrate could repair gut dysfunction, by promoting regulatory T cell (Treg) differentiation and/or accumulation and mediating anti-inflammatory activities [119–121] or inhibiting histone deacetylases (HDACs), conferring to these metabolites anticancer properties. Butyrate also induced apoptosis in colorectal cancer and lymphoma cells, suggesting direct tumoricidal activities [122, 123]. As a result, a way to prevent or treat cancer would consist in developing dietary regimen that increases intestinal butyrate over acetate production.
27.12 Future Prospects
Over the past decade, microbiome raised a major interest, since it appears that these commensal communities influence the development and outcome of a wide range of disease, including cancer. Microbiota might affect tumor formation and progression. It may also determine anticancer treatment responses. Either indirect effects such as immunosurveillance or direct effects of microbial compounds (i.e., carcinogens, cytotoxic agents, and metabolites) may affect tumor cells via diverse processes (mutagenesis, epigenetic modulation, stimulation of receptors on host cells, effect on anabolic and catabolic pathways).
Given that the cancer immune set point may be largely controlled by the health of our gut microbiome, diagnosis tools to evaluate a patient’s gut dysbiosis are urgently needed to be able to guide the indications of specific therapies and anticancer probiotics. Technologies based on culturomics, metagenomics, or PCR or mass spectrometry will lead to diagnosis tools for cancer-associated dysbiosis and predictors of primary resistance to ICB.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree