Fig. 2.1
Disease-free survival (Panel a) and relapse-free survival (Panel b) during 25 years of follow-up after surgery among women with clinically negative axillary nodes and women with clinically positive axillary nodes. There were no significant differences among the groups of women with negative nodes or between the groups of women with positive nodes in either analysis. Printed with permission from NEJM
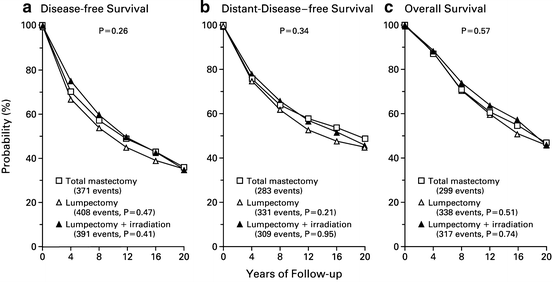
Fig. 2.2
Disease-free survival (Panel a), distant-disease-free survival (Panel b), and overall survival (Panel c) among 589 women treated with total mastectomy, 634 treated with lumpectomy alone, and 628 treated with lumpectomy plus irradiation. In each panel, the P value above the curves is for the three-way comparison among the treatment groups; the P values below the curves are for the two-way comparisons between lumpectomy alone or with irradiation and total mastectomy. Printed with permission from NEJM
Conclusion: These observations from the NSABP trials provide good evidence that the long-term outcome of the breast cancer patients will be determined by whether the treatment is given early or late in the natural history of the disease. Had the NSABP trial results been correctly interpreted, both the mammography screening trials and the NSABP trials would have arrived at the same conclusion, as follows: The current therapeutic regimens are most effective at an earlier stage of breast cancer, when the probability of systemic metastases is lower.
2.
Fisher’s “alternative theory” also implies that finding non-palpable breast cancers at screening will not lead to a decrease in breast cancer death, but the large volume of evidence, including their own, does not support this theory.
Results from randomized controlled trials: To date there have been ten randomized controlled mammography screening trials (eight population based) which tested the influence of early detection upon the disease-specific mortality from breast cancer. Meta-analyses of these trials have shown a highly significant, long-term mortality benefit from invitation to screening [10, 13, 14]. Very-long-term follow-up (29 years) of the largest of the mammography screening trials showed a highly significant 31 % decrease in mortality from breast cancer in the women invited to screening compared with the uninvited control group (relative risk [RR] = 0.69; 95 % confidence interval [CI]: 0.56–0.84; P < .0001). This long-term evaluation also demonstrated a steady increase in the absolute benefit of early detection, in terms of the number of lives saved, which continued well beyond 20 years of follow-up (71 lives saved at 10 years, 141 lives saved at 20 years, 158 lives saved at 29 years) [21]. Thus, the majority of the benefit of mammography screening occurs more than 10 years after screening begins. The more aggressive cancers would have led to breast cancer death in the first 10 years without early detection and surgical removal, while some of the more slowly growing, “indolent” cancers would have led to death after 10–20 years of follow-up in the absence of screening. Claims that mammography screening finds mostly “indolent” cancers [26–28] fail to acknowledge published evidence documenting the propensity for a dedifferentiation of the tumor malignancy grade. The term “ultralow risk tumors” is thus unrealistic and misleading [27].
Results from evaluation of service screening: It should be noted that the randomized controlled trials use the “intention-to-treat” approach, which includes all women with breast cancer, both those who attended and those who declined the invitation to screening. Mortality from breast cancer is decreased to a greater extent in women who attended screening regularly than in the invited group as a whole. Disease-specific mortality among the women who attended screening regularly has been quantified in several ongoing service screening programs. A highly significant reduction of 43 % was observed in Sweden and 49 % in the Netherlands in women who attended mammography screening regularly [15, 19]. This emphasizes the importance of distinguishing between the effect of invitation to versus the effect of regularly attending mammography screening [20].
The issue of subgroup analysis: The population-based randomized controlled trials were all designed to have sufficient statistical power to evaluate the impact of early detection on mortality from breast cancer within the age group selected. However, when the populations were inappropriately subdivided into age cohorts of unequal size (40–49 vs. 50–69), the younger, smaller cohort with lower breast cancer incidence had insufficient statistical power. The resulting lack of a statistically significant decrease in mortality within individual age subgroups was erroneously interpreted as evidence of no impact at ages below 50 years, despite the existence of clear trends towards fewer advanced tumors and decreased mortality. Meta-analysis of trials shows a significant mortality reduction with the policy of offering screening in women aged 40–49 [10, 29]. Also, when Sweden gradually implemented nationwide screening, the option for the lower age limit was either 40 or 50 years. As it happened, the individual counties independently chose 40 years as the lower age in approximately half of the country. This gave the opportunity to evaluate the impact of screening in a population aged 40–49 which was sufficiently large for statistical significance, comprising more than 16 million women-years with 16 years of follow-up. A highly significant 29 % decrease in breast cancer mortality was documented in the women who attended screening (RR 0.71; 95 % CI, 0.62–0.80). This reduction occurred in a country where treatment guidelines are uniform and closely adhered to, so this mortality reduction was achieved in addition to the benefits of modern therapeutic advances [17].
3.
All these results have convincingly demonstrated that the diagnosis and treatment of breast cancer at an earlier phase can prevent death from breast cancer, before viable metastases have been developed, confirming that breast cancer is not a systemic disease from its inception, in contradiction to the “alternative theory,” developed by Fisher. The screening trial results unequivocally proved that breast cancer is a progressive disease, and that its progression can be arrested by early detection. As a result, the prognosis of the breast cancer patients can be substantially improved by local treatment. Fisher’s proposal that breast cancer is a “systemic disease from its inception” is either mistaken or, as the screening results convincingly show, it is not relevant to the treatment of node-negative, <15 mm breast cancers. “Screening has made possible the detection of a large proportion of node negative tumors less than 15 mm size (i.e. before the development of viable metastases) and there is substantial evidence that local–regional therapy is effective in these cases and that adjuvant systemic therapy has negligible scope to improve the survival of patients with these tumors; also, the notion of ‘early’ breast cancer for tumors up to 50 mm is clearly outmoded” [22].
Key Points
Mammography screening alters the presentation of breast cancers from mainly palpable to mainly non-palpable. Randomized controlled mammography screening trials have convincingly demonstrated the following:
Early detection through mammography screening and surgical removal at an early phase can prevent death from breast cancer.
Breast cancer is not a systemic disease from its inception. Therefore, when it is detected as either an in situ or a 1–14 mm invasive tumor, it is primarily a surgical disease.
Breast cancer is a progressive disease, but this progression can be interrupted by early detection and treatment at a sufficiently early phase.
The breast cancer patient’s long-term outcome will be mostly determined by whether the treatment is given early or late, rather than by the choice of treatment offered to breast cancer patients.
The revolution in imaging that has enabled the detection of breast cancer at these early stages awaits a similar revolution in histopathology and therapy.
Therapeutic guidelines for screen-detected breast cancers should not be based on trial results obtained from palpable, clinically detected cancers. There is considerable risk for overtreatment when the adjuvant treatment regimens developed for palpable cancers are also used to treat mammographically detected, non-palpable cancers.
Long-term follow up of screen-detected cases is necessary for the accurate quantification of absolute benefit of screening, because the true potential of the so-called indolent tumors to dedifferentiate cannot be accurately predicted at the time of treatment.
The Mechanism by Which Screening Affects the Natural History of the Disease
The randomized controlled mammography screening trials have provided the opportunity to study the mechanism by which earlier diagnosis and treatment affect the outcome of breast cancer. In these trials one group was randomized to receive an invitation to screening, but the other, randomly selected group of women (control group) was not invited. The breast cancers in women invited to screening were diagnosed on average at an earlier phase than the self-detected tumors in the control group. Screening has a significant impact on all three first-generation prognostic factors: tumor size, axillary node status, and histologic malignancy grade. In a high-quality service screening program 15–20 % of the cancers will be in situ and more than 50 % of the invasive carcinomas will be <15 mm in diameter. Early detection also results in significantly fewer cases with axillary lymph node metastases and also prevents worsening of the malignancy grade in a certain percentage of the tumors. Since two components of the TNM classification, tumor size and node status, will improve significantly in women invited to screening, the incidence of Stage II and more advanced cancers will decrease in this same group of women (see Fig. 2.3).
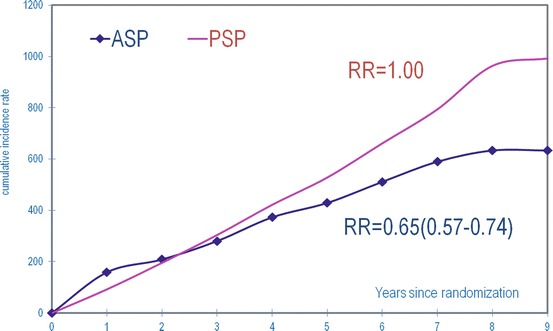

Fig. 2.3
Cumulative incidence rates of advanced breast cancers (Stage II or more advanced) in women invited versus not invited to the Swedish Two-County mammography screening trial. The first screening brought to light both occult and clinically advanced cancers, resulting in the initial slight excess of Stage II + cancers in the invited group. After the first round of screening the advanced cancer rate in women invited to screening fell significantly below that of the control group, because many small invasive cancers were detected at screening and surgically removed before they could grow to a more advanced stage
There is parallelism between the incidence of advanced cancers and the breast cancer-specific mortality rate in any given population, since most breast cancer deaths occur in women whose tumor was at an advanced stage at the time of detection [10, 12, 30–32]. Thus decreasing the incidence rate of advanced tumors through screening will result in a corresponding decrease in breast cancer mortality in this same group of women. In the Swedish Two-County Trial the advanced cancer rate began to fall starting from year four and onwards in women invited to screening, as did the breast cancer death rate. Both of these declines were a consequence of early detection and treatment in an earlier phase (see Fig. 2.4).
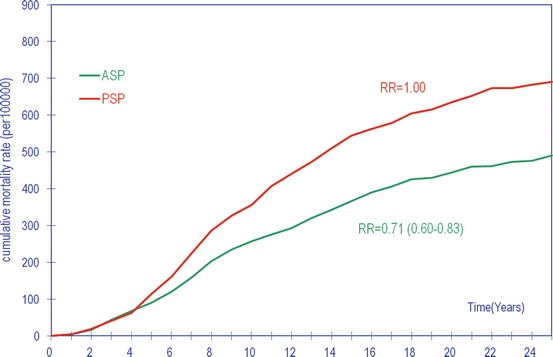

Fig. 2.4
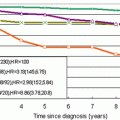
Cumulative breast cancer mortality in women invited to mammography screening (ASP) compared to women not invited (control group, PSP) at 25-year follow-up after randomization
The effect of tumor size on long-term survival (28 years) in the Swedish Two-County Trial is presented in Fig. 2.5. The beneficial impact of screening is reflected in the excellent long-term survival of women with in situ and 1–14 mm invasive breast cancer. The 28-year survival according to axillary node status and distant metastases for all tumor sizes demonstrates the profound prognostic impact of these parameters (see Fig. 2.6). These survival rates are from the era prior to the widespread use of chemotherapy for primary breast carcinoma; none of the women with <20 mm node-negative tumors received chemotherapy in the Swedish Two-County Trial (1977–1985). Women with lymph node metastases had significantly poorer survival than those without lymph node metastases. The cumulative survival of women aged 40–69 years according to the histologic grade of the invasive cancers is shown in Fig. 2.7.
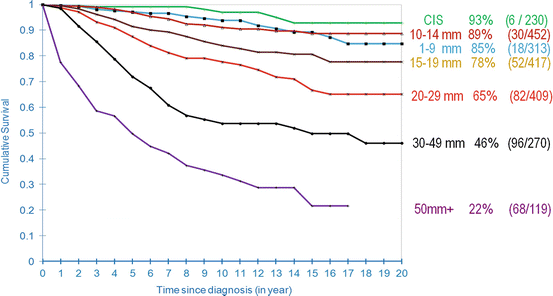
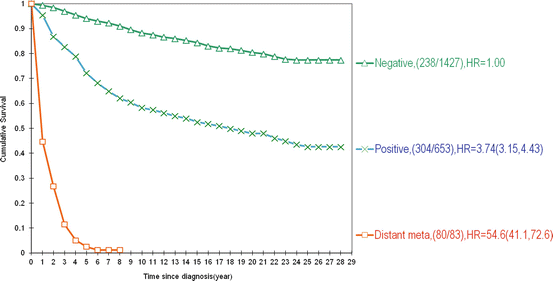
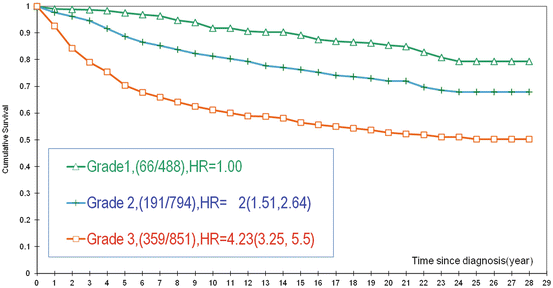

Fig. 2.5
20-year disease-specific survival of women according to tumor size in Dalarna County, Sweden

Fig. 2.6
28-year disease-specific survival of women according to axillary node status and the presence of distant metastases in the Swedish Two-County Trial, for all tumor sizes

Fig. 2.7
Cumulative survival of women aged 40–69 years according to the histologic grade of invasive breast cancers from the Swedish Two-County Trial, for all tumor sizes
Many breast tumors display intratumor heterogeneity, containing two or more histologic types and phenotypes (see Fig. 2.8a–d) [33–35]. Early detection through screening prevents many small, well- or moderately differentiated tumors from developing into more poorly differentiated, larger tumors. The evidence that the histologic malignancy grade worsens as the breast cancer progresses comes from the analysis of both clinical [36] and screening data [37]. The clinical research of Tubiana et al. demonstrated that “during their growth tumors progress towards higher grades” [36]. Duffy et al. used data from a randomized controlled mammography screening trial to perform a more precise measurement of progression by comparing the tumor characteristics in the control group, where the tumors were allowed to grow until clinically detectable, with the tumor characteristics in the group of women invited to mammography screening, where screening aimed at arresting tumor growth [37]. This comparison required the removal of the prevalence screen tumors from both groups in order to eliminate length bias. These two sets of tumors were then equivalent in all aspects except that the tumors in the group invited to screening were diagnosed, on average, earlier. Comparison of tumor size, node status, histologic malignancy grade, and detection mode showed that the proportion of cancers with positive nodes and a higher malignancy grade increased with increasing tumor size [3, 37]. There were also significantly fewer node-positive and poorly differentiated cancers among women invited to screening. There could be two competing explanations for these results: (1) The malignancy grade remains unchanged as the tumor grows, and screening has mostly detected well- and moderately differentiated tumors (length bias sampling). (2) The malignancy grade tends to worsen as the tumor grows, and tumor progression does indeed occur. If this were to happen, one would see a deficit of poorly differentiated tumors in the incident cancers of a group of women invited to screening compared with an uninvited group. When Duffy et al. eliminated the length bias cases from both groups, i.e., the prevalent screen, and could thus compare the incident cancers in those invited and those not invited to screening, they observed that the tumors were significantly smaller and there was a significant deficit of poorly differentiated tumors in the invited group. This demonstrated that screening prevented the deterioration of the malignancy grade of some of the tumors. In summary, tumor progression (worsening of the malignancy potential through the process of dedifferentiation) has been shown to occur in both clinical and screening studies [36, 37].
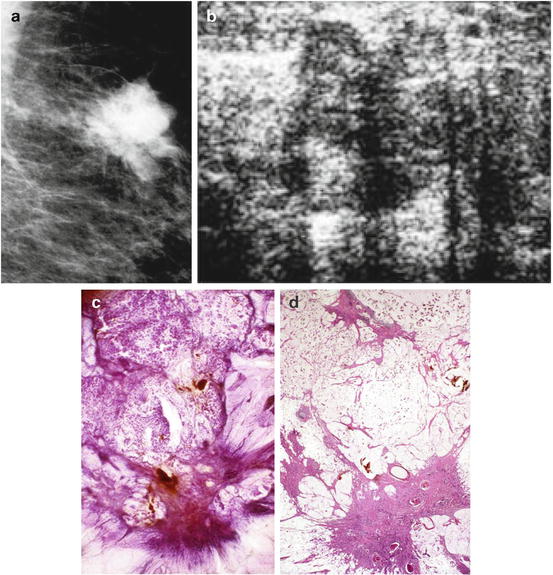

Fig. 2.8
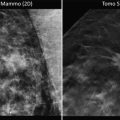
Details of the mammographic (a) and ultrasound (b) images of a tumor containing both a circular/lobulated and stellate components. Subgross, 3D (c) and large thin-section (d) histology images show that the stellate component corresponds to a moderately differentiated invasive ductal carcinoma and the lobulated component is a well-differentiated mucinous cancer
Detailed analysis of the breast cancer cases from women invited and not invited to a randomized controlled trial demonstrated that the rate of poorly differentiated breast cancer increases in all age subgroups with increasing tumor size, but in premenopausal women this process of dedifferentiation occurs more rapidly, earlier in the preclinical detectable phase, and to a greater extent than in postmenopausal women. All these factors in combination make it necessary that women are invited to screening at a frequency which takes into account the varying tumor growth rates according to different histologic tumor types and women’s age [29, 38]. Reversion to less frequent screening, as recommended by the US Public Services Task Force (USPSTF), would tend to increase the number of advanced (more frequently poorly differentiated and node positive) cancers at the time of treatment and increase fatality from breast cancer [39].
The recent publications by Esserman et al. maintain that screening does not decrease the incidence of advanced breast cancers [27, 28]. This is contrary to the published evidence [10, 12, 30–32, 40]. The claim of Esserman et al. that “tumor biology does not change over time” [27] reflects unfamiliarity with appropriate statistical analysis of clinical [36] and screening trial data [37] and fails to account for certain fundamental observations in breast tumor biology, including the consequences of intratumor heterogeneity [35].
Key Points
Breast cancer screening has a favorable impact on all three first-generation prognostic factors: tumor size, node status, and histologic malignancy grade.
The favorable prognosis of women with screen-detected breast cancers can be accounted for by smaller tumor size, less node positivity, and lower malignancy grade at the time of treatment.
The frequency of poorly differentiated breast cancers increases with increasing tumor size.
The frequency of node positivity increases with increasing tumor size.
More frequent screening will reduce the number of interval cancers, and also improve the prognostic characteristics of screen-detected cancers.
Multifocal and Diffusely Invasive Breast Cancers: High Fatality Rate and High Recurrence Rate
Our primary goal is to reduce mortality from breast cancer. Mammography screening and the associated improvements in diagnosis and therapy have enabled us to reduce the breast cancer mortality in women attending screening regularly by 40–50 % [19]. Despite this accomplishment, women are still dying from breast cancer. Investigation into the characteristics of the cancers that are still causing breast cancer death requires assessing the extent of the disease as a measure of the tumor burden. Two comprehensive whole-breast histologic studies examined the unifocal, multifocal, and diffusely infiltrating nature of breast cancer [41, 42]. The term multifocality includes (a) multiple in situ cancer foci without invasion, (b) a solitary invasive carcinoma associated with multiple in situ foci, and (c) multiple invasive breast cancer foci with or without associated in situ cancer. The invasive cancers with or without an associated in situ component are responsible for breast cancer death; the relative frequency of unifocal/multifocal/diffusely infiltrating invasive breast cancers is approximately 68/27/5 % [109]. In which of these groups is breast cancer most fatal? The fatality ratio of unifocal breast cancers (with or without associated in situ foci) is 9.1 % and most (74 %) of these fatal cancers were >2.0 cm in size. In the era of mammography screening enhanced by the use of multimodality breast imaging, unifocal tumors can be detected and successfully removed before they reach the size of 2.0 cm. The fatality ratio in multifocal and diffusely infiltrating invasive breast cancers is 20 and 26 %, respectively, considering all sizes of tumors [43]. Multifocality is an important, independent negative prognostic factor (see Fig. 2.9) and its harmful effect becomes more significant with increasing tumor size. Weisenbacher and coworkers have arrived at the same conclusion [44].
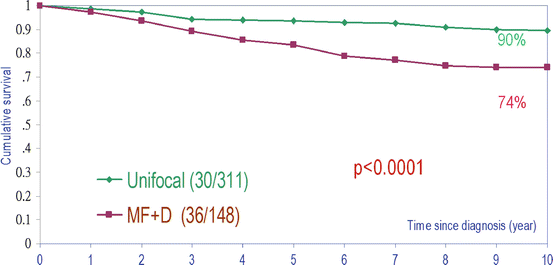

Fig. 2.9
Cumulative survival of women with unifocal invasive versus combined multifocal and diffusely infiltrating breast cancer
The highly significant size-related survival difference also applies to multifocal breast cancers, suggesting that a combination of imaging methods that enables detection of multifocal cancers with a lower tumor burden (when the largest tumor focus is <15 mm) will result in a lower fatality rate (see Fig. 2.10). The multimodality approach (mammography, automated breast ultrasound, and especially breast MRI) will detect multifocal cancers having a lower tumor burden, and will correspondingly lower the fatality rate. This emphasizes the importance of using breast MRI to determine the presence and extent of multifocal disease. The use of breast MRI in multifocal and diffusely infiltrating invasive breast cancers is invaluable in describing the true extent of the disease. This is an important part of treatment planning to prevent incomplete resection of breast cancer at primary surgery. Incomplete resection of invasive cancer foci is associated with a poor outcome: “For patients who underwent second surgery, the finding of a residual invasive carcinoma was associated with increased risk for distant recurrence (22.8 vs. 6.6 %; HR 3.5; 95 % confidence interval, 1.8–7.4; P < .0001).” These same authors concluded, “there is a need to improve techniques for the presurgical and/or intraoperative determination of margins” [45].
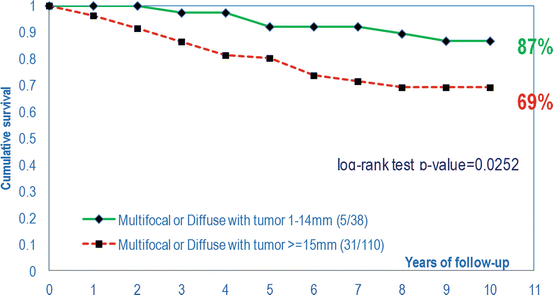

Fig. 2.10
Cumulative survival of women with 1–14 mm multifocal invasive versus >15 mm multifocal invasive breast cancer
Modern, high-resolution breast MRI has the capability of describing the true extent of the disease in the vast majority of cases, far exceeding that of earlier MRI technology, on which most currently available reviews are based. The COMICE trial, which used 2.4/4.0 mm slice thickness (as opposed to the current practice of 0.7–1.0 mm), was a multicenter trial in which 45 centers supplied an average of only 18 cases each during the 5-year accrual period starting in 2002. This study’s failure to detect an impact of preoperative MRI upon reoperation rate may reflect the outdated technology and the extremely low average rate of patient accrual per site, reflecting limited experience in breast MRI interpretation. High-resolution breast MRI was practically nonexistent prior to 2007. For these reasons the COMICE trial results [46], the meta-analysis by Houssami et al. [47], and other earlier studies may have lost their relevance to current breast MRI practice.
The reliance upon local recurrence as a measure of success or failure of breast cancer treatment is subject to serious limitations. Fatality often occurs without local recurrence and the term “local recurrence” as used in the literature does not discriminate among recurrences in unifocal, multifocal, and diffusely infiltrating breast cancers. One classification system uses a cutoff point of 4.0 cm to separate “extensive” from “non-extensive” breast cancer [42]. Using this arbitrary cutoff point, “A disease extent ≥4 cm was shown to be an independent marker for local recurrence; the cumulative 10-year local relapse rate for the group with a disease extent ≥4 cm was 20.5 %, and for the rest 6.7 % (p value = 0.003)” [48].
The seriousness of multifocal and diffusely infiltrating breast cancers has not been generally appreciated for two main reasons. First, the current TNM classification system does not account for multifocality, using only the size of the largest invasive focus as the major descriptive factor. This seriously underestimates the actual tumor burden of multifocal tumors. We have proposed a quantitative evaluation of tumor burden in terms of total tumor volume and surface area [43].
Second, the current practice of histopathology of breast specimens has serious limitations; “conventional techniques may not reflect the extent of neoplasia when the neoplasia is impalpable or grossly indistinct as in the case of dense breast tissue.” Additionally, “complete specimen examination is rarely performed in clinical practice.” “In a typical 8-cm diameter lumpectomy specimen, assuming four conventional pathology margin sections are removed in a single plane, only 16 % of the circumference is examined microscopically” [49] (see Fig. 2.11). “People blame MRI instead of the limitations of conventional pathology and a failure of small section pathology to correlate with MRI and mammography.” (Lee Tucker, M.D., F.A.P.C., personal communication 2012).
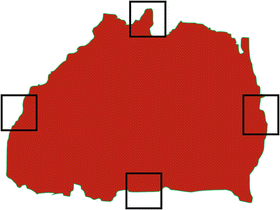

Fig. 2.11
Conventional pathology samples only 16 % of the circumference in a typical 8 cm lumpectomy specimen (courtesy of Lee Tucker M.D., F.C.A.P.)
We recommend that large-section histopathology should be standard for all breast cancer surgical specimens, as it also provides better correlation with breast imaging (see Fig. 2.12a–m).
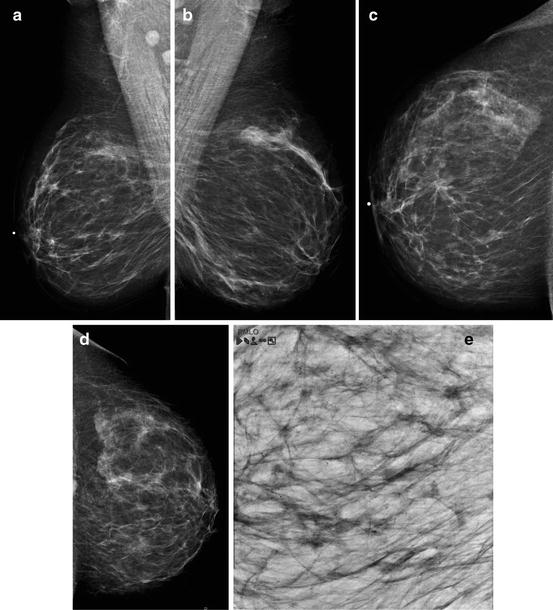
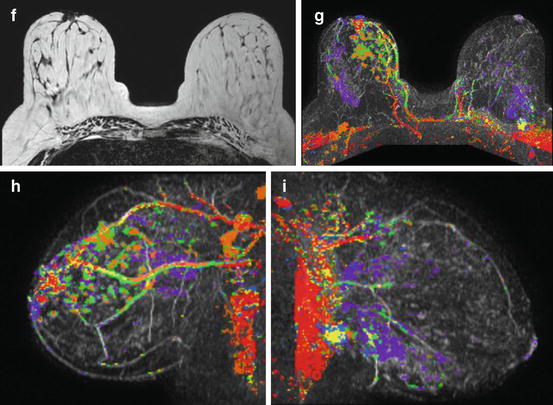
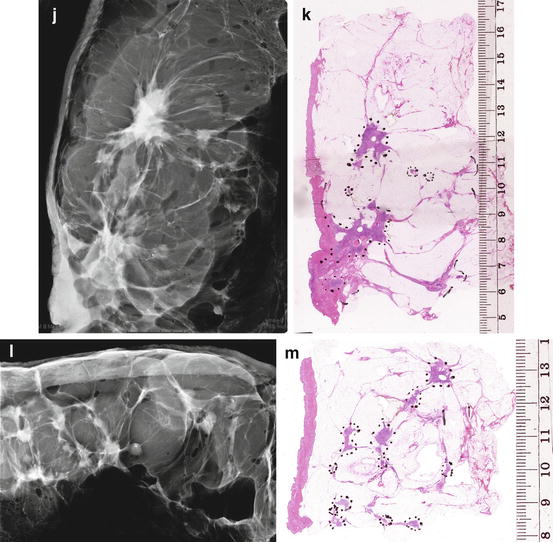



Fig. 2.12
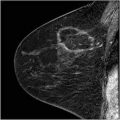
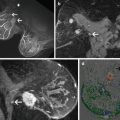
(a–e) This 49-year-old woman felt a lump under her right areola. A slight degree of skin retraction could be provoked over the tumor. Physical examination confirmed the presence of a hard tumor, but also revealed a “thickening” in the upper and central portions of the right breast. The mammograms show a retroareolar asymmetric density corresponding to the palpatory finding. In addition, there are a large number of small stellate lesions spread throughout the upper-medial portion of the right breast, pathologic lymph nodes in the right axilla, and an oval tumor mass in the upper portion of the left breast. (f–i) Breast MRI of the right breast shows at least 30 independent tumor foci in the upper-medial portion of the breast with washout pattern (histologically proven invasive breast cancer foci) and a solitary, oval lesion with benign features in the left breast (histologic examination of the core biopsy specimen: fibroadenoma). (j–m) Correlation of the right mastectomy specimen slices with large-section histology. Multifocal cancer: at least 25 invasive tumor foci (well and moderately differentiated), the largest measuring 33 mm × 15 mm, and the smallest focus being 8 mm. The second and third largest foci measure 20 mm × 12 mm and 10 mm × 8 mm. In addition LCIS and Grade 1 and 2 in situ carcinoma are found over a 45 mm × 11 mm area. Six out of 17 surgically removed axillary lymph nodes showed metastases at histologic examination
Key Points
Despite the remarkable improvements in the diagnosis and therapy of breast cancer that resulted in a significantly decreased mortality from the disease, it is unfortunate that women are still dying from breast cancer.
The fatality rate is highest for multifocal and diffusely infiltrating breast cancer cases and lowest for unifocal tumors.
Multifocality is an important, independent negative prognostic factor whose harmful influence increases with increasing tumor size.
Even multifocal invasive breast cancers can be detected in a relatively early phase with a lower tumor burden and a correspondingly lower fatality rate, provided that the most sensitive imaging methods are used preoperatively. The combination of currently available imaging methods, especially breast MRI, has this capability.
The use of preoperative MRI helps to prevent incomplete resection of breast cancer at primary surgery because it provides more accurate determination of tumor size and extent than either mammography or breast ultrasound.
The failure to remove invasive breast cancer foci is associated with a poorer outcome.
The current TNM classification system should be upgraded to provide a better quantitative evaluation of the tumor burden by categorizing unifocal, multifocal, and diffusely infiltrating breast cancers separately.
Large-section histopathology of all breast cancer surgical specimens should be the standard of care.
The Need for Improved Terminology Reflecting the Site of Origin of Breast Cancer
Breast cancer originates either from the epithelial cells lining the acini within the terminal ductal lobular unit (TDLU) or from the cells lining the milk ducts (see Fig. 2.13). The majority of breast cancers originate from the TDLUs, not from the ducts. Figure 2.14 shows the relative distribution of the crushed stone-like and powdery microcalcifications on the mammogram, both of which are the mammographic presentations of in situ tumor growth which arise from and are localized within the TDLUs. Despite the fact that these in situ tumors arise within the lobules and not from the ducts, they are paradoxically termed “ductal” carcinoma in situ. When this population of cancer cells invades the surrounding breast tissue, forming a stellate or circular/oval-shaped tumor mass, the invasive tumor is also erroneously called “ductal.”
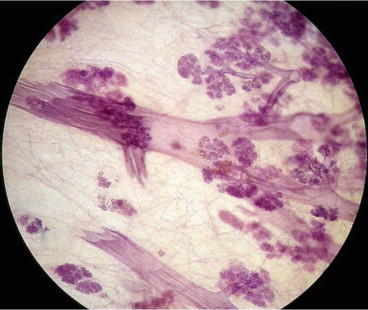
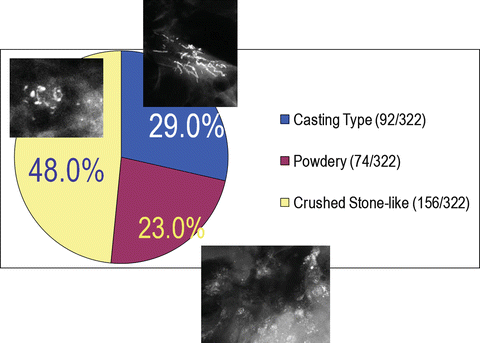

Fig. 2.13
3-Dimensional histology image of major milk ducts and several terminal ductal lobular units (TDLUs). The majority of breast cancers originate from the TDLUs

Fig. 2.14
Relative distribution of histologically proven calcified in situ carcinoma cases according to their presentation on the mammogram. The crushed stone-like and powdery microcalcifications constitute the majority of in situ cases. Both of them are the mammographic presentations of in situ tumor growth arising from and localized within the TDLUs
In situ breast cancers are usually detected at mammography. There are more than ten distinctly different mammographic presentations of in situ cancer subtypes (see Figs. 2.15a –2.15c and 2.16a–x), but current terminology bundles them all under the same name: DCIS. This simplification unfortunately leads to misunderstanding and confusion. Additionally, the term DCIS is a misnomer, since the vast majority of in situ carcinomas do not arise from the milk ducts and are not situated within these ducts.
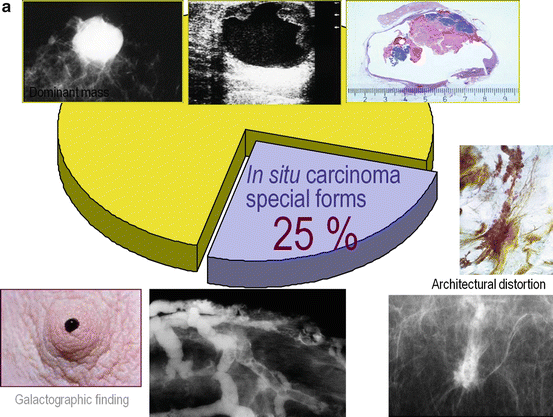
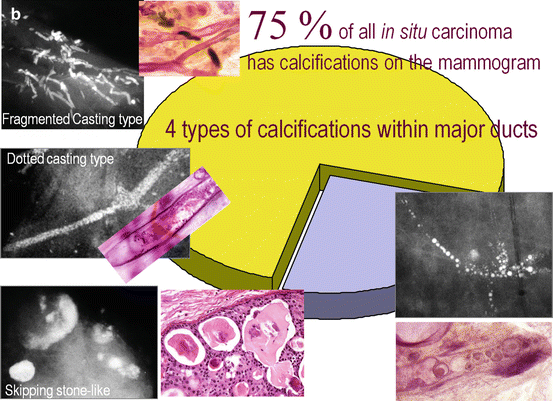
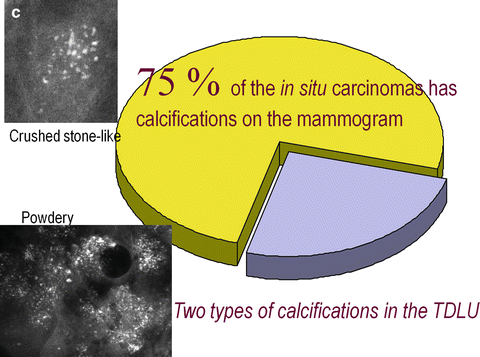
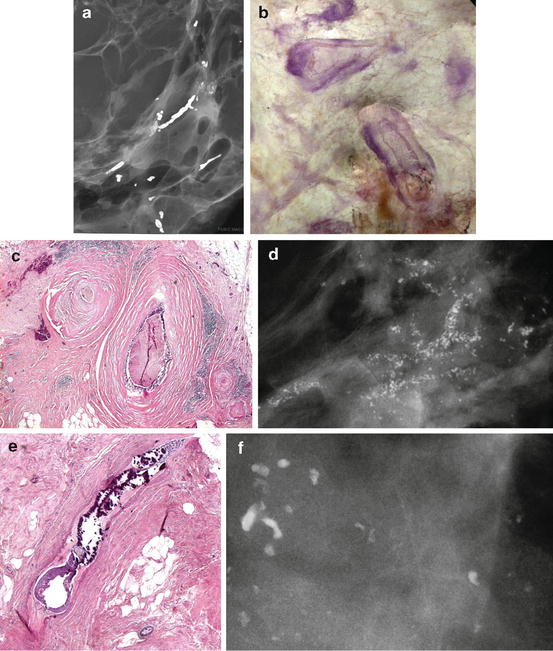
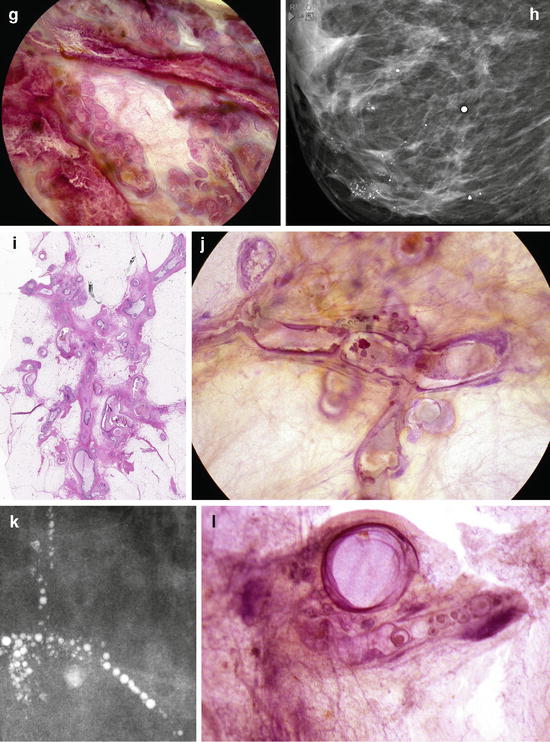
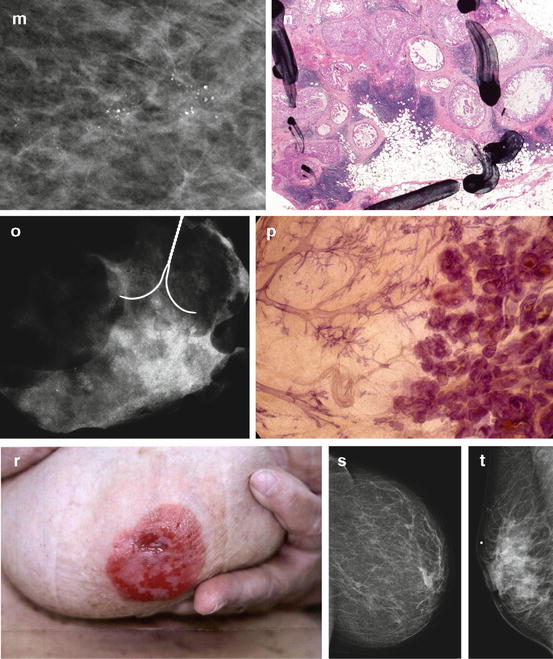
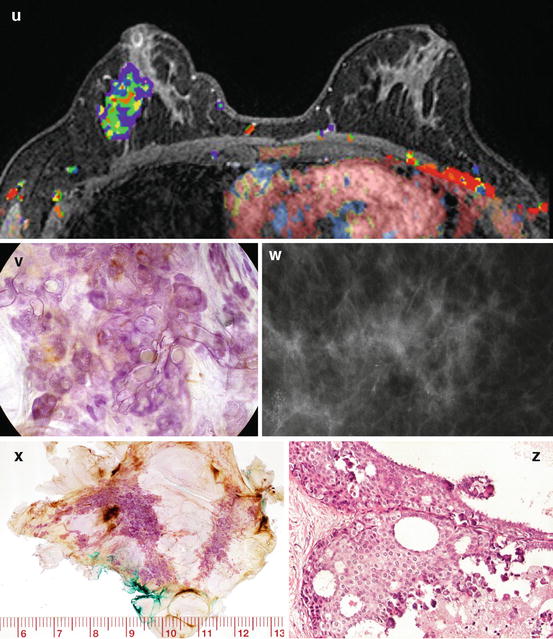



Fig. 2.15
(a) About 25 % of the mammographically demonstrable in situ carcinomas lack calcifications on the mammogram. In these cases the mammogram shows either a dominant mass or a architectural distortion; the third option is a galactographic finding. (b) In 75 % of the Fig. 2.15 (continued) mammographically demonstrable in situ breast cancer cases calcifications are seen on the mammograms. There are four different mammographic appearances of the calcifications associated with the malignant processes localized within the major ducts. (c) In 75 % of the mammographically demonstrable in situ breast cancer cases calcifications are seen on the mammograms. There are two different mammographic appearances of the calcifications associated with the malignant process localized within the terminal ductal lobular units (TDLUs)




Fig. 2.16
A collage demonstrating the mammographic and histologic heterogeneity of in situ carcinoma of the breast. Regrettably, there is still only one term in current use to describe all these different diseases, and that term is “DCIS.” (a–c) Fragmented casting-type calcifications in Grade 3 in situ carcinoma with solid cell proliferation. (d, e) Dotted casting-type calcifications seen in high-grade carcinoma in situ with micropapillary cell proliferations and necrosis within the major ducts. (f, g) Skipping stone-like calcifications seen in high-grade carcinoma in situ with micropapillary cell proliferations without necrosis, but with fluid production in the major ducts. (h–j) A mixture of crushed stone-like and skipping stone-like calcifications spread over two-thirds of the right breast. The Grade 2 and 3 in situ carcinoma contiguously fills the major ducts and branches as well as a large number of TDLUs. (k, l) Pearl necklace-like calcifications: Grade 1 in situ carcinoma with cribriform cell architecture and large psammoma body-like calcifications in the major ducts. (m, n) Multiple clusters of crushed stone-like calcifications localized within TDLUs: Grade 2 in situ carcinoma with solid cell proliferation, central necrosis, and amorphous calcifications in Fig. 2.16 (continued) the extremely distended acini. (o, p) Multiple clusters of powdery calcifications: Grade 1 in situ carcinoma associated with psammoma body-like calcifications in the TDLUs. (r, s) Paget’s disease. In this case the mammogram is normal, and the high-grade in situ carcinoma was occult for mammography. In most of the Paget’s disease cases the mammograms show malignant-type calcifications within the major ducts. (t–v) Palpable tumor and architectural distortion with no associated calcifications on the mammogram. The histology shows a large number of cancer-filled, tortuous ducts with high-grade micropapillary cancer in situ, no necrosis, and extreme fluid production. (w, x, z) Architectural distortion associated with calcifications within the cancer-filled, distended, tortuous ducts
Breast cancers actually arising within the major milk ducts have a histopathologic appearance (see Fig. 2.17a, b) very similar to that of metastatic prostate cancer (Fig. 2.17c–e) and metastases of breast cancer to the axillary lymph node(s) (Fig. 2.18a, b). Although the histopathologic appearance shown in Figs. 2.17c–e and 2.18a, b will be termed by pathologists as invasive cancer, i.e., when found in the prostate or in the axillary lymph node(s), the similar histopathologic appearance is termed “DCIS” when found in the breast. The unpredictable clinical course and also the occasional fatal outcome of these cases indicate that, contrary to its name “ductal carcinoma in situ” of the breast, the special breast cancer subtype originating from the major ducts may behave as an invasive cancer and can prove fatal (see Figs. 2.19, 2.20, and 2.21) [50].
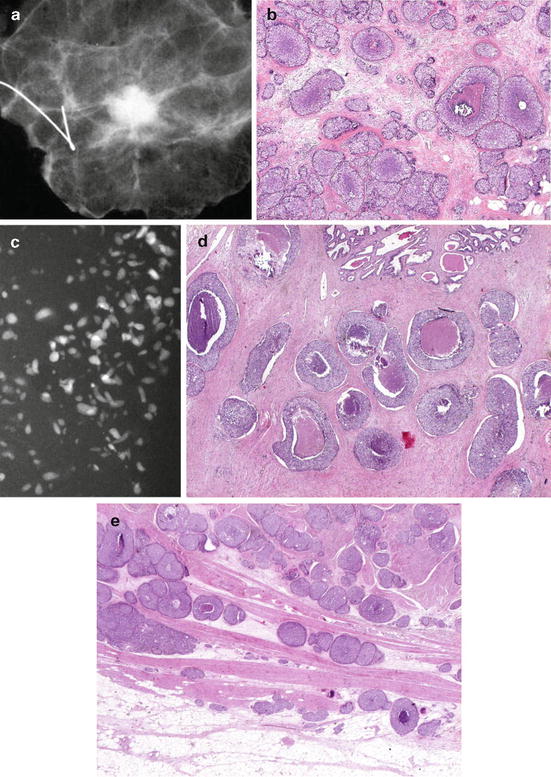
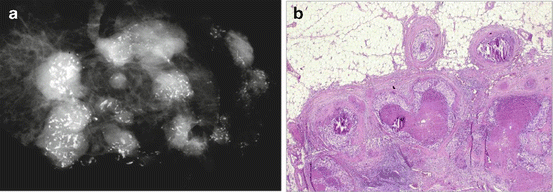
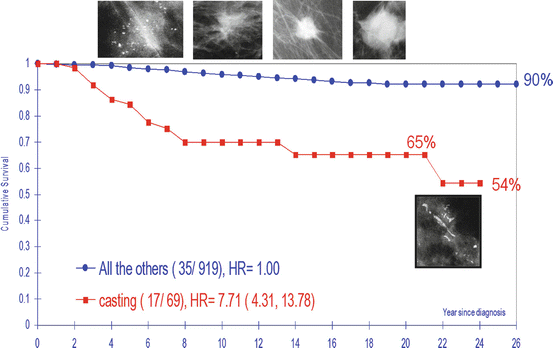
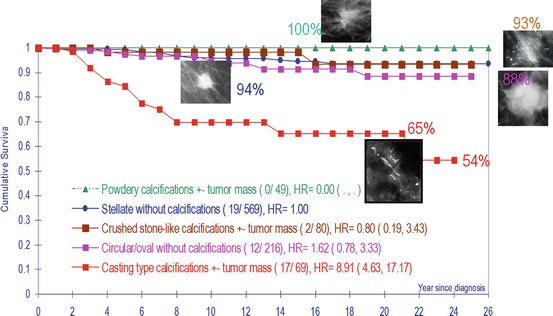
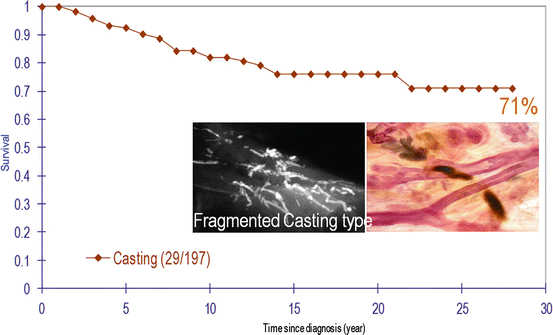

Fig. 2.17
(a, b) Segmentectomy specimen radiograph containing breast cancer. The histology image (b) is very similar to the histology of the prostate cancer shown in (d). (c) Specimen radiograph of a prostate cancer (ductal adenocarcinoma of the prostate DAP). (d) Intermediate power histology image of this prostate cancer. (e) This DAP infiltrates the surrounding organs in the lesser pelvis; the cancer-filled ducts can be seen among the muscle fibers of the urinary bladder

Fig. 2.18
(a, b) Radiograph of an axillary specimen containing 12 pathologic lymph nodes with malignant-type calcifications. The histology of one of the axillary lymph nodes contains “duct-like structures,” mimicking the histologic image of prostate cancer (DAP) shown in Fig. 2.17d and the so-called in situ breast cancer shown in Fig. 2.17b

Fig. 2.19
26-year cumulative survival of women aged 40–69 years with 1–14 mm invasive breast cancers by mammographic tumor features. Dalarna County, Sweden. 1–14 mm invasive breast cancers originating from the TDLU (AAB) have excellent (90 %) long-term survival, compared to the subtype of ductal origin (DAB), presented on the mammogram as casting-type calcifications (65 % long-term survival)

Fig. 2.20
Cumulative survival of women aged 40–69 years with 1–14 mm invasive breast cancers as a function of the five mammographic tumor features

Fig. 2.21
Cumulative survival of breast cancer cases with casting-type calcifications on the mammogram. Women 40–69 years old, diagnosed in Dalarna county, Sweden, between 1977 and 2006
Taking the logical and consistent nomenclature that is used to describe prostate cancer and using it to describe breast cancer as well can resolve these terminological inconsistencies and the resulting confusion. Our proposed terminology emphasizes the site of origin of the cancer: acinar adenocarcinoma of the prostate (AAP) would correspond to acinar adenocarcinoma of the breast (AAB), in which the cancer originates from the TDLU. Similarly, ductal adenocarcinoma of the prostate (DAP) would correspond to ductal adenocarcinoma of the breast (DAB), in which the breast cancer originates from the major milk ducts. The striking difference between the long-term outcome of breast cancers of similar size originating from the TDLUs (AAB) and the cancers originating from the major ducts (DAB) justifies the radical change in terminology (Figs.2.19 and 2.20).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree