Fig. 2.1
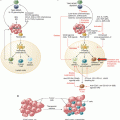
Major immunopathological and genetic events occurring during carcinogenesis. Upon chronic inflammatory stimuli exposure, normal cells undergo transformation into precancerous cells. Local inflammation induces recruitment of myeloid-derived cells that fuel carcinogenesis via production of oxygen derivatives or cytokines. Later on, tumor growth and invasion into tissues are controlled by a balance between antitumor and immune escape mechanisms
Inflammatory mediators are other well-known promoters of genetic alterations. In fact, many of the substances produced by the inflammatory immune cells (such as macrophages and neutrophils) can induce the direct damage of DNA in nonimmune cells. In the presence of noxious stimuli, chronic inflammation can both induce the development of driver tumorigenic mutations and promote the necessary genetic instability to allow other alterations to develop [7]. This process of cancer induced by chronic inflammation (Fig. 2.1) has been described in several pathologies, including gastric cancer in association with Helicobacter pylori infection, asbestos or cigarette smoke exposure and lung cancer, arsenic exposure and skin cancer, gastroesophageal reflux for cancer of the esophagus, inflammatory bowel disease for colorectal cancer, chronic pancreatitis for pancreatic cancer, and pelvic inflammatory disease for ovarian cancer [8].
Examples of inflammatory carcinogenic mediators include reactive oxygen species and matrix metalloproteinases, which can induce DNA damage and extracellular matrix disruption, respectively [9]. In addition, some cytokines can induce the growth of abnormal or preneoplastic cells, such as IL-1β for gastric carcinoma and IL-8 for melanoma. The preneoplastic potential of many other cytokines has also been described (e.g., IL-1β, IL-6, IL-23, and TNF-α).
In virus-related cancers, aside from the inflammation induced by the infection itself, the virus genetic material can integrate into the host genome and induce cell transformation by altering diverse oncogenic pathways [10]. Virus-associated cancers represent roughly 20% of all cancer types and include cervical cancer (induced by HPV), B cell lymphoma (induced by EBV), Merkel cell carcinoma (induced by Merkel cell polyomavirus), hepatocellular carcinoma (induced by hepatitis B and C viruses), and some gastric cancer and H&N cancer (induced by EBV).
2.3 The Tumor Immune Microenvironment
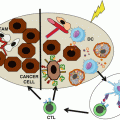
As mentioned above, the tumor microenvironment is a very complex and dynamic ecosystem, where different cellular populations coexist. The major players include tumor, immune, and supporting cells (e.g., fibroblasts, stromal, and endothelial cells) [11]. Immune cells that circulate in the blood enter into tumors via transendothelial migration and are attracted by chemokines produced by tumor cells, fibroblasts, or inflammatory cells. Within the tumor mass, the immune cells locally proliferate, differentiate, exert their functions, and die, and some migrate back to the circulation. Within this population, one often can find cells related to acute inflammation (including neutrophils, basophils, and eosinophils), cells of the innate immune response (including macrophages, NK cells, and DC), and cells from the adaptive immune response (including cytotoxic CD8+ T cells, Th1-/Th2-skewed T cells and B cells). We focused this subchapter in the last two populations.
2.3.1 Tumor-Associated Macrophages
Tumor-associated macrophages (TAM) represent an abundant population, and in many tumors they outnumber other immune cells [12]. Although the majority of TAM are found in the invasive margin of the tumor, we can often find also elevated densities within the tumor core [13]. TAMs exhibit an extremely plastic phenotype and function, and two main subtypes have been described: M1 TAM (induced by Toll-like receptor ligands [e.g., lipopolysaccharide and IFN-γ]) which preferentially express pro-inflammatory cytokines and inducible nitric oxide synthase and M2 TAM (induced by IL-4 or IL-13) which express arginase 1, CD206, CD163, IL-4R, TGF-β1, and PDGF [12]. Some works suggest that while M1 TAM potentiate the antitumoral Th1 response and antagonize the suppressive activities of regulatory immune cells, M2 promote angiogenesis, tumor growth, and metastasis [13].
2.3.2 NK Cells
Natural killer cells are cytotoxic effector lymphocytes of the innate immune system whose primary function is to help control infections and tumors [14]. Two major mechanisms of recognition of tumor cells by this population have been described: they can recognize cells which have downregulated major histocompatibility complex class I expression (an immunotolerance phenomenon widely described in many cancer types), or they can bind to stress-induced ligands expressed on tumor cells (e.g., MICA or MICB, which bind to NKG2D expressed on the NK cell) [14].
2.3.3 Dendritic Cells
The main function of dendritic cells (DC) is to establish a bridge between the innate and adaptive immune response. Under physiological circumstances, DC engulf and process nonself-antigens, and when they are exposed to danger or activation signals, they become activated and travel to secondary lymphoid structures in lymph nodes where they prime naïve B or T cells [15]. The DC phenotype is rather plastic, and they can produce a wide range of pro-inflammatory or immunosuppressive cytokines, as well as expressing a large series of activating or inhibition receptors, depending of the environment where they are embedded. The secondary lymphoid organs are protected environments and often provide an ideal milieu to promote a DC phenotype that effectively activates the adaptive immune response [16].
In many cancer types, tumor cells produce molecules that induce pro-inflammatory or tolerogenic DC and block their maturation at different stages. Often, intratumor DCs exhibit an immature and inhibitory phenotype [17]. Interestingly, in recent years, several works have described the presence of tertiary lymphoid structures (TLS) in the invasive margin of many cancer types [18], where in theory the DCs are protected from tumor-produced inhibitory substances and from where they can effectively prime the antitumor immune response [19].
2.3.4 Tertiary Lymphoid Structures
TLS are highly organized lymphoid aggregates that develop in inflammatory pathologies. In cancer, TLS often develop in the invasive margin of the tumors and/or in the stroma and resemble those arising in other chronic infectious or autoimmune diseases [19]. Figure 2.2A illustrates TLS found in clear cell renal cell cancer (ccRCC). Characteristically, TLS exhibit an organization similar to secondary lymphoid organs, including a T cell zone (Fig. 2.2Aa) and a B cell follicular zone (Fig. 2.2Ab), and are often surrounded by high endothelial venules [20]. B cells in TLS form germinal centers; they undergo active proliferative machinery and somatic hypermutation [19]. T cells have a CD62L+/CD45RO+ central memory or a naïve phenotype, and some can be found in contact with mature DC which expresses the DC-Lamp marker (Fig. 2.2Aa) or at the periphery of B cell follicles (Fig. 2.2Ac) [20]. Follicular dendritic cells are also detected forming a network where immune complexes can form and be presented for selection of the high affinity B cells. Plasma cells that produce antibodies are located at the vicinity of TLS [21].
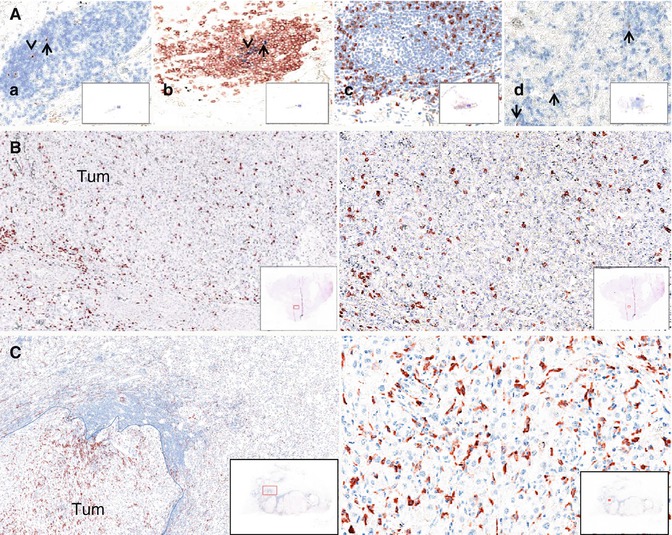

Fig. 2.2
The tumor microenvironment in human clear cell renal cell cancers as detected by IHC on paraffin sections. (A) Tertiary lymphoid structures: (a) DC-Lamp+mature DC (brown) in the CD3+T cell zone (blue); (b) CD20+ B cells (brown) and CD21+ follicular dendritic cells (blue) delineate the germinal center; (c) CD8+ T cells (brown) are distributed around the germinal center; (d) non-TLS-DC-Lamp + DC (brown). (B) CD8 + T cells (brown) (left 5×, right 20×). (C) CD163+ macrophages (red) (left 5×, right 20×), Tum = tumor area
Primary tumors and metastases contain TLS at variable densities, depending on the tumor type and on the patient. As discussed below, it is assumed that TLS reflect the ongoing immune reaction within tumors. They allow the presentation of tumor antigens by mature dendritic cells to T cells leading to the differentiation of CD4+ Th1 cells as reflected by the expression of the T-bet marker and the T-B cell cooperation for B cell differentiation into plasma cells. All of these events can thus occur locally, within the tumor bed. To what extent TLS bypass the need of secondary lymphoid organs to mount or control the antitumor immune reaction remains an open issue.
2.3.5 CD4+ and CD8+ T Cells
CD4+ T-helper cells are divided into different subtypes, including Th1, Th2, Th17, Tfh, and Treg; each subpopulation accomplishes specific roles in the antitumor immune response. Overall, a Th1-oriented response antagonizes the tumor growth and is often associated with good clinical outcome [22]. In fact, Th1-oriented cells potentiate in situ the antitumor function of cytotoxic T cells, through the production of several cytokines including IL-2 and IFN-γ. Tfh cells interact with B cells in TLS, helping antibody production.
The role of other subpopulations of tumor-infiltrating CD4+ T cells (Th2, Th17, and Treg) is less well understood but is often associated with poor prognosis in different tumors [22]. Many studies suggest that Treg in cancer can dampen the antitumor immune response by two main mechanisms: (1) production of inhibitory cytokines (e.g., IL-10, TGF-β, and IL-35) and (2) suppression of DC development and maturation [23].
CD8+ T cells exert a very import function in the antitumor immune response, as they are responsible of tumor cell recognition and elimination. Due to their genome instability, tumor cells often express mutant proteins at their surface. Many of these are neoantigens that can induce a tumor-specific immune response. The primed CD8+ T cells are in charge of the tumor cells recognition and lysis, by mechanisms well described in the literature including the release of cytotoxic granules [24]. Interestingly, in the majority of tumors, infiltrating cytotoxic T cells express inhibitory receptors (e.g., PD-1, Tim-3, and Lag-3), whose function under physiological situations is to contract the immune response upon biding to their ligands. Many tumor cells in fact can take advantage of this inhibitory mechanism and in fact express a wide arrange of ligands (e.g., PD-L1, PD-L2) that help them escape for the T cell attack [25].
2.3.6 B Lymphocytes
In inflammatory settings other than cancer, B cells enhance T cell responses by producing antibodies and stimulatory cytokines and chemokines, serving as local antigen presenting cells and organizing the formation of TLS that sustain the immune response. In cancer, B cell can exert all of these functions and overall have an antitumor effect. In addition, recent evidence suggests they can also play an immunomodulatory role through the production of IL-10, among other cytokines [26].
2.3.7 Spatiotemporal Dynamics of the Tumor Immune Microenvironment
Chemokines ensure the local migration of these different cell types and cytokines allow their cooperation. In addition, many tumors are surrounded by a stroma containing an extracellular matrix composed of fibroblasts that form collagen fibers and produce enzymes––such as metalloproteases––that facilitate local invasion within tissues and ultimately the release of tumor cells that egress to the circulation and migrate in other tissues.
A direct consequence of these processes is that the tumor microenvironment is a tissue-dependent organized structure in which immune cells are common denominators. Figure 2.2B illustrates the presence of CD8+ T cells in the tumoral zone of clear cell renal cell cancer. A closer look into the organization of the immune microenvironment reveals that cells are not evenly distributed in the tumor area. Lymphocytes (T and B cells) are more abundant in the tissue border area called the invasive margin than in the center of the tumor [13]. They can be found dispersed or within aggregates, forming TLS in the invasive margin and/or in the stroma [18, 27]. Most of the T and B cells have a memory phenotype, CD8+ T, CD4+ Treg, Th1, Th2, Th17, and B cells being detected at variable densities, whereas naïve T cells and CD4+ Tfh are exclusively present within TLS. NK cells are detected in the tumor stroma. Some T cells are found in close contact with tumor cells in the center of the tumor. Myeloid cells such as macrophages, myeloid-derived suppressor cells, mast cells, and neutrophils are present at high densities, both in the invasive margin and the center of the tumor. Figure 2.2C illustrates the high density of CD163+ M2-oriented macrophages near the invasive margin of renal cell cancer. Immature dendritic cells are present at low densities, dispersed in the whole tumor area whereas mature dendritic cells are usually found within the TLS, in close contact with T cells (Fig. 2.2Aa). Importantly the immune composition of the tumor microenvironment evolves with the stages of tumor progression in a tumor-dependent manner. Thus, T cells are more numerous at the early stages of the disease in colorectal cancers and at their late stages in renal cell cancers [17, 28]. The density of B cells increases with tumor stage in colorectal cancers, as does that of the myeloid cells such as neutrophils, mast cells, immature dendritic cells, and macrophages. Thus, the tumor microenvironment is a complex structure, forming a tumor-dependent “immune landscape” that evolves during tumor progression.
2.4 The TME Dictates Clinical Outcome for the Patients
Quantification of immune infiltrates and its relationship with prognosis has been studied for more than 20 years. Following the observation that high T cell densities correlate with longer survival in ovarian cancer [29], the Galon, Pagès, and Fridman studies demonstrating for the first time in large cohorts of patients with colorectal cancers (CRC) the association between densities of memory T cells, early signs of metastasis, and patient’s survival made a significant breakthrough in this field [28, 30]. Since then, important progresses in immunohistochemistry (IHC) with the multiplication of robust antibodies, the development of high through put technologies and of automated quantitative imaging has led to numerous studies on immune cell composition of the TME. This real enthusiasm was even more pronounced during the last 5 years with the emergence of checkpoint blockade therapy (CBT), which aims at reversing T cell exhaustion. Thus, T cell abundance in the TME and its link with outcomes and/or response to CBT is under intensive work by many teams worldwide.
2.4.1 T Cells
2.4.1.1 CD8+ T Cells
T cell abundance within the TME has been extensively studied across the majority of tumor types. Our group published in 2012 a comprehensive review of the number of original articles linking immune cell populations infiltrating the tumor and prognosis [11]. We reported that high densities of CD3+ T cells, CD8+ cytotoxic T cells, and CD45RO+ memory T cells were associated with a longer disease-free survival (DFS) and/or overall survival (OS) in most tumors (including melanoma, head and neck, breast, bladder, urothelial, ovarian, colorectal, and lung cancer) [1]. We noted at that time that clear cell renal cell carcinoma (ccRCC) was one of the rare exceptions to the rule. We updated these data last year and found similar results. In addition, we reported new tumor types such as GIST, biliary tract, thyroid, or oropharyngeal cancers where CD8+ cell infiltration was associated with a good prognosis [22].
The poor prognostic value associated with CD8+ T cells in ccRCC was confirmed by our group, both in kidney primary tumors [17] and in ccRCC lung metastases [31]. Besides ccRCC, studies in lung adenocarcinoma [32] and in HCC [33] also reported a poor prognostic value associated with increased CD8+ T cell infiltration, in contradiction with other published studies. In prostatic adenocarcinoma as well, CD8+ T cell densities correlate with poor outcome [34], consistent with our own data [35].
The “Classical” Case of CRC
Colorectal cancer is the archetype of tumors where high CD8+ T cell densities are associated with good prognosis. Indeed a high infiltration of CD8+ T cells, particularly effector memory subtypes (TEM), is correlated with a low probability of metastatic spread and prolonged PFS and OS [28], suggesting T cells may control local invasion in primary tumors and confer a long-term systemic protection against metastasis. Moreover, IHC studies showed that compartmentalization of T cells in the center and the invasive margin of the tumors does matter. An immunoscore (IS) measures the density of CD3+ and CD8+ T cells in the center, and the invasive margin of the tumors has been developed by Jerome Galon’s team and has been validated in a worldwide collaboration approximately 4000 CRC patients [36, 37]. Even if a high T cell density was more frequent in smaller tumors and MSI-positive tumors, the prognostic value of IS was independent from TNM stages and MSI status. Moreover IS was more accurate to predict the prognosis of patients with early stage CRC [37, 38].
The Discordant Case of ccRCC
We recently reported a clear negative association between CD8+ T cell infiltration and outcomes in ccRCC [17]. Within a cohort of 135 patients with available primary RCC tumors, we found that a high density of CD8+ cells, as assessed by IHC, was associated with a shorter disease-free survival and OS. These results were validated for OS in an independent cohort of 51 patients with (resected) lung metastases of ccRCC. The underlying mechanism for this poor prognosis value of CD8+ T cells is not fully understood. We showed that most of the intratumoral T cells have an exhausted phenotype, which may reflect impaired antigen presentation due to the presence of dysfunctional DCs with an immature phenotype (Fig. 2.2Ad). They express the DC-Lamp marker of mature DC but lack the high levels of MHC class II molecules and CD83 expressed by mature DC. They may be involved in the impairment of T cell antitumor response [17]. Consistently, in patients who have a higher density of DC within TLS, a high density of CD8+ was associated with good prognosis. Thus, antigen presentation by mature DC in the TLS seems to be a crucial event to drive antitumor response in ccRCC, in accordance with our previous observations in lung cancers [39]. Moreover, we showed by immunofluorescence (IF) that CD8+ T cells express immunoregulatory receptors such as PD-1 and/or LAG-3, suggesting a highly exhausted phenotype and both associated with poor outcomes [17].
2.4.1.2 CD4+-, Th2-, and Th17-Oriented T Cells
Consistent with CD8+ T cell infiltration, an increased in Th1-oriented CD4 T cell infiltration has been associated with favorable prognosis in almost all tumor types studied including breast cancer [40] or CRC [41].
Prognostic value of other T cell subsets (Th2, Th17) has been far less investigated first because of a low frequency in the majority of the tumors and second because of technical challenges to specifically identify these subsets.
2.4.1.3 Regulatory T Cells (Tregs)
The example of Tregs is eloquent. A high Treg density has been first associated with poor prognosis in ovarian cancer, which has been then confirmed in a variety of tumors such as in breast, lung, melanoma, or colorectal cancers (reviewed in [42]). Nevertheless, other studies reported longer survival associated with high densities of Tregs in colorectal, bladder, head and neck, or ovarian cancers. One of the reasons for these opposite results is the difficulty to identify the Treg population. Tregs are a heterogeneous population that should be ideally identified by a combination of markers (CD4+, CD25+, Foxp3+, T cells). The development of multicolor fluorescence imaging allows to increase the number of cell surface markers for their detection. Beyond the technical challenges, these results highlight that the prognostic impact of immune cell populations depend on the tumor type and on the TME.
2.4.2 B Cells
The positive or negative role of B cells in antitumor immunity has been discussed for many years, mainly supported by mice studies. As compared to T cells, few clinical studies reported the prognostic role of intratumoral B cells. The majority of clinical studies have demonstrated that a high density of B cells within TME is associated with better prognosis including breast cancer [43], NSCLC [21], head and neck cancer [44], ovarian cancer [45], metastatic colorectal cancer [46], biliary tract cancer [47], and primary cutaneous melanoma [48]. Several nonexclusive mechanisms could explain the positive role of B cells in the antitumor immune response, some being antibody dependent by their capacity to trigger complement and antibody-dependent cell cytotoxicity (CDC and ADCC) or to form immune complexes able to activate DCs and others by acting as APC for CD4 [49] and CD8+ T cell immune responses [50]. Indeed, it has been shown that B cells play a major role during initial priming and expansion of CD4+ T cells [51], are able to cross-present antigens to CD8+ T cells [52], and can promote cytotoxic T lymphocyte survival and proliferation [53].
On the opposite, few clinical studies reported a pro-tumoral role of B cells within the TME [54, 55]. B cells may play a pro-tumor function by the maintenance of a chronic inflammation [56], by the promotion of neoangiogenesis [57], and/or by the direct inhibition of cytotoxic T cell responses [55]. Moreover, a subpopulation of immunoregulatory B cells called “Bregs” has been described and has been shown to favor the differentiation and the recruitment of Tregs, thus amplifying the immunosuppressive environment [58].
Beyond the density of B cells, an increasing number of studies reported that the spatial localization of these cells have an impact on patient’s outcome. In particular the density of B cell follicles characteristic of TLS is positively associated with outcomes. M.C. Dieu-Nojean and col. showed that an increase in B cell density within the TLS is associated with prolonged survival in NSCLC patients [21]. Similar results were reported in CRC [59] and oral squamous carcinoma [60].
2.4.3 Macrophages
Tumor-associated macrophages (TAM) are a major component of the TME, found both at the tumor core and the invasive margin. The prognostic value of TAM seems to be dependent of the tumor type. Increased density of TAMs is associated with a good prognosis in CRC [61], HCC [62], prostate [63], and cervical cancer [64]. At the opposite an increased TAM density is associated with poor prognosis in endometrial [65], gastric [66], urothelial [67], HCC [68], melanoma [69], breast [70], ovarian [71], bladder [67], NSCLC [72], and primary CRC tumors [13]. These discrepancies might be explained by the plasticity of these cells since we know that they can switch from a pro-tumoral function (M2) to an antitumoral function (M1) and vice versa [12]. M2 TAMs are associated with a shorter survival and M1 TAMs with a longer survival [22]. Unfortunately, there are no specific or consensual markers to define M1/M2 TAMs. Most of the studies used CD11c or NOS2 for M1 TAMs and CD163, CD204, or CD206 for M2 TAMs, but the use of these markers is still debated.
Tumors contain another heterogeneous subset of cells of myeloid origin, the myeloid-derived suppressor cells (MDSC). Such cells have an immature phenotype and exert profound immunosuppressive activities. Specific and robust tools are still needed for their identification in the human TME.
2.4.4 New Techniques to Estimate the Immune Cell Populations in Tumors
The most broadly used way to quantify tumor-infiltrating immune cells is to detect the protein expression of specific markers either by IHC or IF. These techniques have been improved in the last decade, allowing to detect multiple proteins (multiplex IHC or IF) and to quantify cells automatically. Nevertheless, they remain expensive and difficult to standardize across laboratories, and available antibodies could lack sensitivity or specificity to accurately detect some of immune cell populations.
Efforts have been made to use transcriptome to estimate the composition of the TME. Nevertheless, variability in the signal has limited its applicability until recently. New methods such as CIBERSORT [13] or MCP-counter [73] aim at providing very precise quantitative information about the cell content of heterogeneous samples. Using MCP-counter, we estimated the abundance of immune cells, fibroblasts, and endothelial cell infiltrates, in transcriptomes of 25 different cancers (n = 19,000). The results showed the relative heterogeneity of the cellular composition of the tumor microenvironment in different cancers and confirmed that the inferred density of CD8+ or cytotoxic T cells correlated with favorable prognosis in most cancer types [73] (Fig. 2.3).
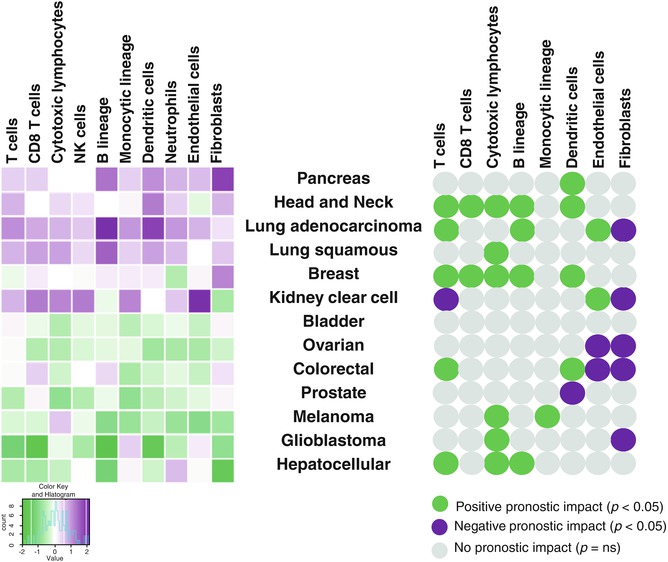

Fig. 2.3
Estimation of the abundance of infiltrating immune and stromal cells and their prognostic significance across human solid tumors. Left, means of MCP-counter scores across malignant tissues (more than 19,000 tumors) in three transcriptomic platforms. Right, univariate prognostic values (overall survival) associated with MCP-counter scores in human solid tumors. Green represents significant favorable prognostic impact and purple significant poor prognostic impact. Gray represents no significant prognostic impact. Adapted from Becht E et al., Genome Biol. (2016) [73]
2.5 TME as Predictors of Response to Therapy
After decades of having targeted on tumor cells and their molecular alterations, new immuno-oncology (IO) agents such as CBT have shed a light on the crucial role of the TME. The currently approved CBT targets are CTLA-4 (ipilimumab) or the PD-1/PD-L1 axis (nivolumab, pembrolizumab, atezolizumab avelumab) [74]. These mAb block the negative signal received by T cells after their interactions with APCs or with tumor cells, thus being able to reverse T cell exhaustion.
As the main target of these agents are T cell infiltrating the tumor, efforts to predict CBT efficacy have been focusing on their characterization in terms of density, localization, phenotype and functionality, before and/or during treatment.
Other well-known and debatable candidates are still investigated as a “biomarker of efficacy” such as PD-L1 expression by IHC or the neoantigen/mutational burden, but are outside the scope of this chapter [75].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree