(1)
Daytona Beach Shores, FL, USA
Constant kindness can accomplish much. As the sun makes ice melt, kindness causes misunderstanding, mistrust, and hostility to evaporate.
– Albert Schweitzer
In previous Chapters, I documented the failure of a five decades-old reliance on the cell-kill approach and more recently of molecularly targeted agents to control advanced cancer. I also identified multiple factors that perpetuate the status quo, documented the commercialization of Oncology practice, and the dehumanization of end-of-life care. Based on that evidence, I advocate a paradigm shift in the treatment of advanced cancer [741]. The shift must be radical but implemented gradually until successful alternatives are developed and in place. The task ahead is momentous but its formulation is beyond the scope of the book. However, some basic practical principles can be envisioned to reach our goal. I refer to the patient-centered principles of beneficence, non-maleficence, respect for patients’ autonomy, and justice that are the ethical foundations of medical care [742, 743].
Simply stated, beneficence is the practice of doing good to others, such as rescuing someone from danger, whereas non-maleficence refers to the ethical principle of “do no harm”, a medical standard inscribed in the Hippocratic Oath. In medical practice, beneficence almost always involves some degree of harm. Hence, caregivers are obligated to balance expected benefits against possible risks of any action, including ordering a test, a procedure, a medication, an operation or a treatment regimen, and ensure that the benefit-harm ratio be favorable. Although physicians are expected to possess the necessary knowledge to make that determination, patients’ autonomy must be respected and any choice of action requires the patient’s acquiescence after full disclosure and a signed informed consent, when appropriate. The policy of obtaining consent is linked to the principle of patient autonomy, even when the benefit-risk ratio of a proposed intervention is most favorable. Justice is perhaps the most controversial ethical principle and the most difficult to apply to individual cases. It refers to the fair adjudication of care between competing claims, which, in modern health care, has become almost synonymous with the fair apportionment of scarce financial resources. While few will challenge these principles’ altruistic goals, their full application in medical practice is offset by the multiple pressures on caregivers and by the needs and beliefs of each individual patient. As a result, ignoring one or more principles of ethical care is a widespread practice that is justified by doubtful arguments in the treatment of advanced cancer and is especially inappropriate at the end-of-life.
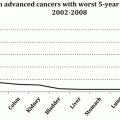
In preceding Chapters, I have shown that, once a diagnosis of advanced or metastatic cancer is confirmed, patients are treated with chemotherapy or more recently molecularly targeted drugs. The issue here is not to question the “raison d’être” of these mostly inefficacious agents, but their often-indiscriminate use that negatively impact patients’ QOL and interfere with a dignified end-of-life without demonstrable benefits. The sequence begins with “first line” drugs or drug combinations chosen among the most efficacious against the targeted tumor. Unresponsive patients and those whose cancer relapses after an initial response are then treated with “second line” regimens that, as the name implies, are even less efficacious but equally toxic. Ultimately, most patients are treated with “salvage” regimes, an approach that rarely salvages anyone. Such a consensus approach to advanced cancer management, fostered by the belief within the medical community that, with powerful tools at our disposal, everything must be done regardless of cost, is a view bolstered by the multiple factors that shape Oncology practice, the emotionally-charged but misleading motto “when there is life there is hope”, and by patients’ attitudes when facing death. Yet, this caregivers’ aggressive stance, clearly justified for the approximately 2 % of curable types, notably trophoblastic cancers (~90 % cure rate), germ cell cancers (~65 %), certain adult and childhood leukemias (~25–75 %), Hodgkin’s disease (~65 %), and certain NHLs (~30 %) [744], induces only marginal survival benefits in most of the remaining 98 %. This is confirmed by the fact that, between 1975 and 2008, cumulative gains in 5-year survival for the 10 most frequent cancer sites that together accounted for 71 % of new cases and 65.5 % of cancer deaths in 2008 exceeded 15 % in only four cancers. Meager as they are, these gains must be credited to new and more efficacious antibiotics for treating chemotherapy-induced infections, easier access to blood product transfusions, and other life-sustaining measures, as much as to treatment of the underlying cancer. Most recent statistics show that patients with ten types of cancer accounting for nearly 50 % of new cases between 2002 and 2008 exhibited a dismal 2–16 % 5-year survival. These grim statistics call into question the value of aggressive practices that violate one or more ethical standards seeking goals seldom attained despite five decades of trying. One recently proposed solution to this predicament, called “personalized medicine”, is based on the notion that clinical trial results are averages of the studied population, which, coupled with the enormous complexity of the disease process, preclude selecting the most appropriate agent for a particular individual. As a result, some patients are under-treated and others are over-treated, and the type and severity of adverse effects vary from patient to patient. However, this seemingly inarguable logic lacks clinical validation, an endeavor that promises to be arduous, long, and costly. Indeed,
Moreover, personalized medicine assumes the efficacy of available drugs for the treatment of cancer, which is not supported by the evidence. Yet, what is even more difficult to explain is the relentless pursuit of aggressive, acute care with renewed vigor at the end of life that, in addition to worsening a gradually deteriorating QOL, ultimately proves futile time and time again. Faced with these realities, it appears clear that a new cancer management paradigm guided by the ethical principles of beneficence, non-maleficence, and justice while respecting patients’ autonomy must be developed. In order to be executed successfully, such a paradigm shift must be strongly endorsed and promoted at the national level by leading public and private health care organizations such as NCI and ACS & ASCO, and supported and implemented by caregivers who hold the key to its success.
In the current climate of increasing molecular testing capabilities, clinicians are urged to approach commercially available biomarker tests with a healthy level of scrutiny of their clinical application and validation. At this juncture, various commercially available assays may be of little added value, and accelerated biomarker development with clinical validation is desperately needed [745].
14.1 Redesigning the Search for New Cancer Agents
While critics will point out that this is already underway through translational applications of newly acquired genome-based knowledge, the current search is conducted largely by individual and secretive pharmaceutical companies more interested in generating high-revenue products quickly than in contributing to the overall control of cancer. The extremely high price of most molecularly targeted anti-cancer agents and the emergence of biopharmaceutical companies dedicated to manufacturing unaffordable orphan agents for treating rare diseases, such as the $410,000 per patient per year price tag of Soliris®, are illustrative. Another example is the accelerated drug approval programs (e.g., Fast Track, Breakthrough Therapy, Accelerated Approval, and Priority Review) launched by the FDA in response to manufacturers’ pressure that benefit, more drug sponsors’ bottom line, than patients’ health. Some will argue that free competition is the best road to better and cheaper products. Although this is certainly true for consumer products, health care is a supply-driven industry where physicians are arbiters of patient needs, making it impervious to market forces that operate in other industries where consumer demand drives supply and keeps costs in check [746]. Nevertheless, drug manufacturers should not be discouraged from participating in the search for better drugs. Yet, theirs are piecemeal efforts targeting specific diseases that are unsuitable as a cancer control strategy. Such a goal can be achieved only through a focused and centrally coordinated national program that, like the Manhattan project (1939–1946), involved 130,000 people to produce the first atomic bomb that ended WWII, and the Apollo Program that within a decade fulfilled President Kennedy’s vision of “landing a man on the Moon and returning him safely to the Earth” when astronauts Neil Armstrong and Buzz Aldrin landed their Lunar Module on the moon on July 20, 1969. While the latter prowess was invoked by Mary Lasker and associates as a key argument for spearheading the movement that led to the National Cancer Act of 1971, I envision a joint national effort towards a single goal and a break with the past at all levels of the cancer research “enterprise”. Such an approach was proposed by Benno C. Schmidt Sr., chairmanship of the President’s Cancer Panel, in his 1971 statement before the Senate Committee on Labor and Public Welfare, “However, there is no comprehensive overall program plan today, and such a plan is a sine qua non of effective assault on cancer” [747]. For instance, the practice of awarding research funds to individual researchers to support their pet projects promotes, by design, a fragmented rather than cooperative approach to the War on Cancer. On the one hand, while the motto “one brick at a time” is frequently invoked in cancer research circles to support such an uncoordinated approach, it is more a catchy expression than a means to an end, as the War on Cancer demonstrates. On the other hand, “study section” members at granting agencies who review and recommend individual projects for funding are themselves researchers with expertise in the subjects assigned to them whose decisions are often questionable. This is illustrated by my own experience. Two interrelated applications for research funds were rejected by NCI; one to develop CLL and/or HCL cell lines (“not doable” I was told); the other to study our own anti-cCLLa1 MoAbs [748] aimed at future human trials (“MoAbs are too dangerous for human use”). These projects, subsequently funded by the Department of Veterans Administration, led to the development of two HCL cell lines [749], their in vitro and in vivo characterization [750], and that of four MoAb-derived immunotoxins [751, 752]. If not biased, my reviewers’ decision revealed a spectacular lack of vision. Indeed, a myriad of MoAbs, in one form or another, are currently part of our armamentarium for treating numerous diseases ranging from rheumatoid arthritis to cancer. To replace this disjointed and multidirectional approach, I propose enrolling tens of thousands of scientists in the medical, biological, biochemical, and other complementary fields of science, concentrated in a few campus-like environments to join forces and focus their interdisciplinary expertise towards a common goal of unraveling the secrets of cancer development and progression and developing tools for their mastery. Participants within each field would bring to the table expertise known or potentially relevant to cancer. Data generated within each field would be analyzed at pre-determined intervals and interpreted in the context of data generated in other fields to serve as a new point of departure for further collaborative study. Such an approach is more likely to uncover common underlying processes that lead to cancer development and progression and the means to block or reverse them than the current fragmented and uncoordinated hit-and-miss approach of developing and screening individual drugs aimed at individual types of cancers; an old technique pioneered by Paul Ehrlich in his 7-year quest for antimicrobials one century ago.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree