Fig. 7.1
The Venus of Willendorf
Even a few decades ago, it was still deemed desirable. Townsend et al. [2] nicely documented that “certain ethno-cultural groups associate large body sizes with marriageability, attractiveness, fertility, and generosity … traditionally, some Pacific Islanders associate power and status with large body sizes and that …. Pacific Islanders with higher Body Mass Indices (BMI = kg/m2), compared to Whites with higher BMIs, were more likely to see themselves as either under or normal weight.”
The concept that being large adds to a powerful image is not limited to the Polynesian islands. Winston Churchill, Nikita Khrushchev, Louis XVIII, and many other international leaders come to mind. It is only recently that obesity in a prominent person, such as Governor Christie, has become a disadvantage, enough to cause him to undergo the insertion of an adjustable gastric band.
7.3 No, Severe Obesity Is a Disease!
There were others, however, who recognized long ago that obesity was detrimental to health. Four hundred years before the birth of Christ, Hippocrates noted that “If we could give every individual the right amount of nourishment and exercise, not too little and not too much, we would have found the safest way to health.” Shakespeare had Falstaff complain “Thou seest I have more flesh than another man, and therefore more frailty” and described Hamlet as “fat and scant of breath.” Kintz [3] probably summarized our change of thought most concisely, “Obesity isn’t as cool as it used to be, back in the earlier centuries. Before it was a reflection on your gross income, and now it’s just gross.”
Prior to the 1950s, what is currently known as bariatric surgery did not exist. In 1954 Kremen [4] and colleagues made the first critical step with the recognition that severe obesity is a disease and that diets, exercise and medications were ineffective in almost all of the patients. Led by their clinical observation that extensive resection of intestine could lead to severe weight loss, they pursued experiments in dogs that demonstrated that by excluding 50–70 % of the small intestine, they could produce profound weight loss.
This conceptual breakthrough was followed and the origins of bariatric surgery later applied to human subjects through the development of what is known as the jejunoileal bypass (JIB) (Fig. 7.2). Payne built upon the work done in the 1950s by Kremen and developed his version of the JIB which was actually a jejunocolic shunt and identified the associated metabolic changes 1963 [5]. Payne’s original procedure involved the division of the small intestine 35–50 cm downstream from the ligament of Treitz. The proximal end was then anastomosed to the proximal transverse colon. Both the jejunoileal and jejunocolic bypasses achieved the goal of weight loss and lowered serum cholesterol due to the decreased absorption of dietary fats. The one black mark on the history of metabolic surgery is that over 30,000 of these operations were performed with multiple reports of severe malnutrition, mineral imbalances, diarrhea with 8–12 bowel movements/day, perianal excoriations, hepatic cirrhosis, hepatic failure, renal stones, severe dehydration, hypocalcemia, and poor vitamin absorption [6, 7] before Griffin, in a scathing editorial, demanded these procedures be abandoned.

Fig. 7.2
Jejuno-ileal bypass
Even so, there were some successful cases and, for the first time, there was evidence that a surgical approach, if it was the right approach, could overcome severe obesity. That recognition and the drawbacks of the JIB procedure and its variations spurred innovative surgical options to safely achieve weight reduction in morbidly obese individuals. By the 1970s the jejunoileal bypass had been essentially abandoned and attention was being directed towards a different approach.
The finding by Buchwald [8] that exclusion of the terminal ileum could provide long-term control of hyperlipidemias needs to be noted as well as a sentinel signal that the metabolic effects of intestinal surgery deserved investigation.
7.4 The Currently Accepted Operations
The design of the current operations was led by Mason, appropriately often referred to as “the father of metabolic surgery” who documented, in a series of thoughtful and minutely recorded studies, that operations on the stomach and proximal small bowel, could achieve weight loss with far greater safety and better outcomes. In 1967 Mason and Ito [9] developed the original gastric bypass . Their configuration consisted of a horizontally oriented proximal gastric division with creation of a 12 mm loop gastrojejunostomy anastomosis. Issues related to marginal ulceration as well as significant bile reflux sparked many variations to the gastric bypass procedure culminating into the Roux Y configuration by Griffen [10] with a more vertically oriented pouch that represents the standard procedure that is performed today. Mason also developed the operations to limit intake with a small gastric pouch and a small outlet, i.e. the vertical banded gastroplasty (VBG) that rose to prominence in the early 1980s. At that time it rivaled the Roux-en-Y gastric bypass; however it ultimately fell out of favor as many of the patients that underwent this procedure ultimately experienced significant weight regain. The VBG procedure consisted of the creation of a vertical pouch with use of a circular stapler to create a gastric window followed by a non-cutting linear stapler to form a small vertically oriented pouch. The pouch itself is formed on the lesser curve side of the stomach and a narrowed stoma is created between the pouch and the distal stomach after application of a silastic band. The VBG was a modification of the horizontal gastroplasty procedure as described by Printen and Mason [11].
In the meantime, in Italy, Scopinaro [12] pursued a series of detailed and rigorous studies that led to the development of the most effective metabolic operation, i.e., the biliopancreatic diversion (BPD) procedure, which he described in 1979. And, as Mason, he did far more than design an operation. He followed his patients with great care, conducted demanding clinical trials and reported his results with clarity and candor. The BPD still stands today as the most effective procedure in terms of weight loss and comorbidity resolution with remission rates of type 2 diabetes in the 92–95 % range. The concern is that the greater exclusion of gut from contact with food may be too radical with its harsh restrictive and malabsorptive characteristics [13]. The current version of his BPD consists of a horizontal gastrectomy, which leaves 200–500 mL of proximal stomach. The duodenal stump is then closed. A gastrojejunostomy with a 250 cm roux limb is subsequently created. The long biliopancreatic limb is then anastomosed to the roux limb at a distance of 50 cm proximal to the ileocecal valve. This operation although rarely performed in the USA is thought to be an appropriate procedure for the obese individuals with BMI’s >60 kg/m2.
Complications including a perceived higher incidence of protein calorie malnutrition, marginal ulceration and perforation provoked modification of Scopinaro’s procedure and led Hess [14] and colleagues to add a duodenal switch procedure to Scopinaro’s BPD. The duodenal switch procedure was initially described by Dr. DeMeester [15] as a potential treatment for severe duodenogastric reflux. Hess successfully theorized that by adding this modification to the original BPD, the beneficial aspects of the BPD could be retained while eliminating its unwanted complications.
The biliopancreatic diversion with duodenal switch (BDP-DS ) has been performed in the USA over the last 27 years. Key differences as it relates to the BPD include a 100 cm common channel (as opposed to 50 cm) and a different type of partial gastrectomy. The gastrectomy is created by removing the greater curvature of the stomach (i.e., sleeve gastrectomy). It is commonly done over a 40–60 French bougie. The antrum, pylorus and proximal duodenum are preserved. This effectively provides a greater restrictive component by leaving a smaller gastric reservoir (150–200 mL) as compared to the original BDP. The duodenum is divided distal to the pylorus and a duodenojejunostomy is created between the proximal duodenum and a 150 cm roux limb. Both the BPD and BPD-DS are currently performed totally laparoscopically by highly skilled minimally invasive surgeons [16, 17]. Even so, that approach still led to more nutritional complications than the RYGB and is not widely used although there are champions who feel it is the best metabolic operation.
During the late 1990s the Roux-Y gastric bypass had become the most widely performed bariatric surgical procedure. RYGB is considered to be the “gold standard” of bariatric surgical procedures due to its effectiveness with weight loss and its acceptable complication profile [18]. The procedure involves proximal gastric pouch formation, roux limb formation, and performance of a gastrojejunostomy. Proximal pouch formation involves creating a 15–30 mL gastric pouch by transecting the stomach (usually with a stapling device). The Roux limb is created by transecting the jejunum at a point 15–75 cm distal to the ligament of Treitz. An end to side jejunojejunostomy is then made 70–150 cm down the roux limb. Long limb bypass with roux limb segments greater than 150 cm are performed by some surgeons which imparts a significant malabsorptive component to the operation [19]. The gastrojejunostomy is then fashioned by bringing the Roux limb alongside the gastric pouch without tension. This may be done in a retrocolic, antecolic, retrogastric, or antegastric configuration. The particular configuration chosen is largely surgeon dependent with conflicting data available regarding stricture and leak rates [20, 21]. Construction of the gastrojejunostomy anastomosis can be performed using one of several techniques. It can be created using a linear stapler, a circular stapler, or completely hand-sewn. There are studies available in the literature that support the use of each of the mentioned techniques [22].
Sleeve gastrectomy had its beginnings as a component of the BPD-DS operation [23]. It is now being performed with increasing frequency as a stand-alone procedure in the treatment of morbid obesity. The technique is relatively novel and therefore has not yet been standardized in all of its steps (bougie calibration size, staple line reinforcement method, and type of stapler used) [24]. The longitudinal gastrectomy typically begins with dissection in gastrocolic ligament along the greater curvature of the stomach. This is carried all the way up to the level of the angle of His. A calibration bougie is then placed and advance along the lesser curve through the pyloric channel. The stomach is then divided with sequential firings (4–6) of an endo-GIA stapling device alongside the bougie. The remnant stomach is then extracted from the largest port site which completes the procedure. Laparoscopic sleeve gastrectomy represents a relatively simplistic yet effective alternative for bariatric surgery candidates.
Gastric banding is a restrictive technique used to treat morbid obesity that was introduced into clinical practice in the 1980s. The current version of gastric banding was popularized by Dr. Kuzmak [25] and involves the placement of an adjustable silicone band around the upper portion of the stomach. This technique effectively creates a small gastric pouch with a narrow stoma. The stoma diameter is adjusted to its proper size by injecting saline into a subcutaneously placed port that is connected to the silicone band via a long silicone tube. This procedure is done laparoscopically and is considered the least extensive of the bariatric surgery procedures that are currently performed. The procedure consists of proximal stomach dissection through either a pars flaccida or perigastric technique to create the pathway through which the band is passed and secured around the stomach. Most recently the perigastric technique has been largely abandoned because of higher posterior slippage rates [26].
Laparoscopy was first applied to metabolic surgery by Belachew with adjustable gastric banding [27]. This was shortly followed by Wittgrove with the game-changing demonstration that the complex Roux-en-Y gastric bypass (RYGB) could also be performed with this approach [28]. Subsequent to these developments many large series were published on the safety and efficacy of the laparoscopic approach to bariatric surgery [29].
The application of a robotic platform to assist in performing complex bariatric operations has gained some interest in recent years. Advocates of robotic bariatric surgery cite advantage when compared to laparoscopic surgery including (1) removal of counter-intuitive motion and instrument tremor, (2) decrease in the physical demands of the operating surgeon, (3) 3-dimensional visualization, and (4) increased flexibility and degree of movement is inherent in the robot arms [30]. To date all contemporary bariatric surgery operations have been successfully performed using robotic technology with comparable results to that of laparoscopy [31–34].
7.5 Measurement of Outcomes: Are the Claims Really True?
If the surgeons were startled by the unexpected result of metabolic surgery, their medical colleagues and, in fact, the public met the reports with disbelief. How could a simple intestinal operation cure diabetes, cut mortality by values as high as 80 % and even prevent solid cancers? It was just not possible! The reports were dismissed as just other previous surgical claims of miracles such as sympathectomy for peripheral vascular disease and removal of the carotid body for hypertension. The initial high mortality rates and epidemic of liability suits only confirmed the general impression that metabolic surgery was a hoax.
What was needed was at least a long rigorous clinical observational study of a large group of patients who had undergone a standardized operation. Our group at East Carolina University was well suited for such a study. The 29 counties we serve in the rural part of eastern North Carolina hold an underserved and impoverished population in the USA who tended not to leave the region and who were burdened with a prevalence of severe obesity four times higher than in the rest of the USA. In that setting, we pursued the study of a standardized “Greenville gastric bypass” with a 30 mL. gastric pouch, a 1.0 cm gastrojejunostomy and a retrocolic 60 cm alimentary limb. From 1980 to 1996, the study included 837 consecutive patients with a follow-up of 93 % with a mean of 9.2 years.
We could not duplicate that study today because the rules for human research have changed so sharply. In those years our approach was not only legal and appropriate but it also benefited the patients. We promised the participants (1) free care for their families, including their children, if the complied with follow-up. That action became illegal with congressional ruling that one could not charge the US Government more than a private individual. Continuing that course would have made us ineligible to bill for Medicare. (It also stopped the time-honored practice of “professional courtesy,” i.e., the practice of giving free care to ministers, nuns, nurses, and colleagues.) We also had (2) a driver who picked the participants up at home and drove them to the clinic in a university van. That practice, also appreciated by the patients, ceased with the university attorney’s ruling that the legal liability was too great. Finally, (3) when we lost a patient to follow-up due to a change of name or address, we would seek the help of the sheriff who would provide location and transport. That approach would certainly violate the rules of HIPAA today but, in fact, the patients loved the free transportation and the officers were glad to perform duties that were not dangerous. This is not an argument against the protection of human subjects, which we support strongly, but rather an explanation on how we were able to achieve such results. The NIH study, i.e., the “Longitudinal Assessment of Bariatric Surgery (LABS)” shows that a 92 % follow-up over time is still possible but so expensive that the NIDDK had to cancel it after only 7 years.
The data from these studies extending over 16 years were invaluable in documenting that the gastric bypass produced durable weight loss of >100 lbs. with an 8 % regain over the following years and that at the point where the mean follow-up was 9.2 years, the remission rate of type 2 diabetes was 83 % (Fig. 7.3) [35]. In addition, we noted the reduction of other comorbidities of the metabolic syndrome including hypertension and hyperlipidemias. Finally, these studies documented a reduction in mortality of 78 % in the diabetic cohort.
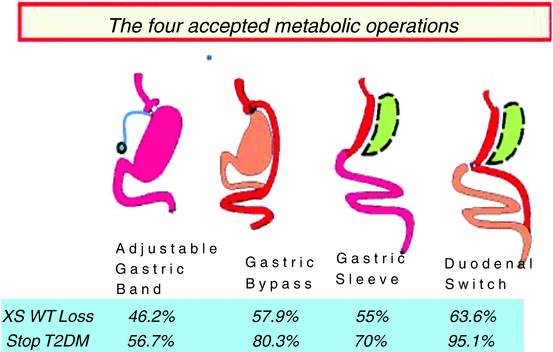

Fig. 7.3
Four accepted metabolic operations
Since then, there have been ample corroborations of these observations, especially by the Swedish Obesity Group, the NIDDK funded study, “Longitudinal Assessment of Bariatric Surgery (LABS), the Surgical Review Company’s “BOLD” database, the NSQIP data base of the American College of Surgeons. There are variations in the data depending on the measures for full remission of diabetes, levels of blood pressure, duration of the disease, racial groups and differences in the operations but, overall, the data are all in accord in documenting the full and durable weight loss, remission of diabetes and comorbidities with a reduction in mortality. In addition, and still unexplained, is the reduction in the prevalence of solid cancer of about 65 % within 5 years [36].
7.6 Bariatric Surgery Centers of Excellence: The Pursuit of Safety
With the growing epidemic of obesity and diagrams depicting the surgical operations as just new variations of intestinal surgery, the procedures were quickly adopted by surgeons but too often with high morbidity and mortality rates.
The severely ill posed major technical challenges and the hospitals, the anesthesia services, the consulting medical colleagues and the support staff were not prepared for the difficulties involved in caring for these patients. Most were hypertensive, diabetic with limited pulmonary and cardiac reserve and, as in the development of cardiac surgery, the initial patients were also the sickest. Basic nursing equipment such as large wheel chairs, beds and adequate stretchers were frequently unavailable. Resources for imaging were not large or strong enough to address the problems of patients weighing over 400 lbs. Anesthesiologists had difficulties intubating these patients and calculating dosages for the anesthetic agents and fluids. Many surgeons were not familiar with operating and caring for patients of this size. Sometimes the available instruments were not long enough to reach the diaphragmatic hiatus in open operations. Most important, members of the nursing staff were not yet familiar with the lack of abnormal vital signs and the subtle warnings that were the first findings of an anastomotic leak in these immunoincompetent patients. And no one was prepared with the rapid deterioration of these patients who could progress from a mild discomfort to a fatal problem in a matter of hours. Accordingly there were major disparities in morbidity and mortality rates among institutions with mortality rates in some hospitals that exceeded ten percent. Intensive care units were strained with the care of complications due to anastomotic leaks and sepsis. Public skepticism concerning the safety of bariatric surgical procedures grew rapidly. Carriers refused to cover the operations, malpractice suits exploded, and liability insurance premiums became unaffordable. It became the “perfect storm” that led to widespread denial of the only effective life-saving therapy for the severely obese.
In 2003, it was evident that this crisis needed to be addressed. Accordingly, the officers of the American Society for Bariatric Surgery (Drs. Champion, Pories, and Wittgrove) founded the ASMBS Centers of Excellence program to recognize those hospitals with the best outcomes. With the nationwide support of the bariatric surgeon, they not only required data with full reporting of outcomes but also set demanding criteria that included the requirement for the presence of experienced surgeons, reporting of all bariatric cases within 24 h of surgery, hospital facilities that were fully equipped to care for these challenging cases, availability of the full breadth of relevant consultants and a well-trained hospital staff (www.surgicalreview.org) [37]. In addition, a landmark conference was held at Georgetown University in 2005 where world leaders of bariatric surgery convened to help provide insight and consensus on what the standards of excellence should be. To assure quality control, the data were confirmed by site visits conducted by nurses experienced in the care of bariatric patients. Initially, the program was to be managed by the ASMBS but the Society was warned by counsel that such an arrangement would not have the credibility of an independent entity. Further, it would make the ASMBS vulnerable to legal actions by hospitals denied certification [38].
To avoid these concerns, the ASMBS founded the Surgical Review Corporation [38, 39] which, under the leadership of Gary Pratt, grew the program to include 425 hospitals and 22 countries. The goal of such a program would be to improve the outcomes of bariatric surgery and reduce the overall expense for bariatric surgery patients (including cost related to complications and comorbid conditions) [38]. To keep track of the data, the corporation developed a software program, the Bariatric Obesity Longitudinal Database (BOLD), a computer based entity that not only listed the presence or absence of a comorbidity but quantified these diseases. For example, the query on diabetes offered five possible answers: (1) no diabetes, (2) diabetes controlled by diet, (3) diabetes requiring the use of oral agents, (4) diabetes requiring the use of insulin, and (5) uncontrolled disease. Certification also required completion with each operation and visit within 24 h, affording access to data in real time. That approach allowed close tracking. For example, it became possible at the end of a day to determine how many patients had diabetes, how many were insured and the number of and reasons for readmissions.
This process was well received by the bariatric surgical community and subsequently implemented. The results were impressive. In a matter of just 2 years, the hospitals who would not commit the effort to become centers stopped providing metabolic surgery and the 90 days mortality dropped to 0.3 % throughout the USA. That figure comes into focus when compared to the mortality rates of other common operations: coronary artery bypass graft, 2–4 %; colectomy, 4–6 %; pancreatectomy, 6–10 %. The only common procedures that match bariatric surgery in safety are routine cholecystectomies and hip replacements. To emphasize the comparison even further, the 90-day mortality for normal deliveries in the USA is 0.1 %. These are remarkable figures given the fact that patients who undergo bariatric surgery are usually grade III anesthetic risks due to diabetes, hypertension, cardiopulmonary disease, immune-incompetence, and mental health challenges. Analyses of these data confirm that surgeons meeting centers of excellence requirements produce better outcomes than those who do not adhere to these standards [40–42].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree