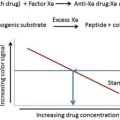
The administration of intravenous heparin to postoperative patients by Barritt and Jordan reduced the incidence of fatal and nonfatal pulmonary embolism and established heparin as the standard for parenteral anticoagulation. The coumarin family of vitamin K antagonists quickly became the standard for long-term oral anticoagulation. Aspirin became a widely used antithrombotic agent after the discovery that chronic oral administration reduced the incidence of secondary strokes and myocardial infarction. This article gives a brief history of antithrombotic therapy, including the discovery of heparin, the vitamin k antagonists, and the utility of aspirin.
Key points
- •
The administration of intravenous heparin to a small number of postoperative patients by Barritt and Jordan dramatically reduced the incidence of fatal and nonfatal pulmonary embolism and established heparin as the gold standard for parenteral anticoagulation.
- •
The coumarin family of vitamin K antagonists quickly became the standard for long-term oral anticoagulation after 3 months of therapy dramatically reduced the recurrence of deep venous thrombosis in patients who had previously received a few weeks of intravenous heparin.
- •
Aspirin, which was a venerable antipyretic and antiinflammatory agent, became a widely used antithrombotic agent after the discovery that chronic oral administration reduced the incidence of secondary strokes and myocardial infarction by 25%.
Having practiced medicine for almost a half century, with a focus on coagulation disorders and antithrombotic therapy, I have become quite familiar with the classic antithrombotic drugs: heparin, warfarin, and aspirin. No one can dispute their effectiveness. The administration of intravenous heparin to a small number of postoperative patients by Barritt and Jordan dramatically reduced the incidence of fatal and nonfatal pulmonary embolism and established heparin as the gold standard for parenteral anticoagulation. The coumarin family of vitamin K antagonists quickly became the standard for long-term oral anticoagulation after 3 months of therapy dramatically reduced the recurrence of deep venous thrombosis in patients who had previously received a few weeks of intravenous heparin. Aspirin, which was a venerable antipyretic and antiinflammatory agent, became a widely used antithrombotic agent after the discovery that chronic oral administration reduced the incidence of secondary strokes and myocardial infarction (MI) by 25%. Even modest doses, as low as 81 mg daily, permanently inhibit platelet cyclooxygenase and, thereby, for a few pennies a day, effectively reduce the recurrence of cerebral and coronary artery thrombosis and embolism.
Of course, no drug is perfect and each of these agents has a downside. One universal problem is that all of the antithrombotic agents marketed to date increase the risk of bleeding. Each of these drugs also has unique side effects. Unfractionated heparin is immunogenic and causes thrombocytopenia and platelet activation in 10% to 15% of recipients. The vitamin K antagonists require frequent laboratory monitoring to assess drug dosing and meticulous attention to dietary intake of vitamin K, as well as monitoring of interactions with other drugs that can either enhance or reduce anticoagulant activity. The management of patients taking a drug like warfarin is so complex that most large institutions have had to invest in a physician-supervised anticoagulant management service staffed by experienced nurses and pharmacists. They do so to enhance patient safety despite that insurers do not reimburse for this valuable service.
Aspirin is, perhaps, the easiest drug to administer, although there has been concern among cardiologists that some patients may be resistant to the agent. Most cases of resistance are due to noncompliance or to simultaneous ingestion of other nonsteroidal antiinflammatory drugs (NSAIDs), such as ibuprofen or sodium naproxen, which compete with aspirin and prevent it from irreversibly acetylating its most important target, platelet cyclooxygenase. A small number of patients do require more than the now standard antithrombotic dose of 81 mg per day and might be called resistant, at least to a baby aspirin. Doubling the dose usually overcomes the resistance.
What is remarkable about the discovery of heparin and the vitamin K antagonists, and the observation that aspirin has antithrombotic properties, is the serendipity and empiricism that led to these discoveries. Very little was known about the intricacies of blood coagulation or the genesis of thrombi when heparin and the vitamin K antagonists were discovered. The pursuit of aspirin as an antithrombotic involved a large leap of faith taken by a humble general practitioner. Moreover, by today’s standards for drug development, neither the vitamin K antagonists nor heparin would garner Food and Drug Administration (FDA) approval, despite their long and illustrious histories as antithrombotic agents. As we enter a new era of antithrombotic therapy with modified heparin molecules, direct oral anticoagulants and the possibility, for the first time, of selective agents that may inhibit thrombus formation without increasing the risk of hemorrhage, it is worthwhile taking a look back to see how these 3 legacy antithrombotic agents were discovered and how they have been used to treat patients with thromboembolism because they provide the rock on which the exciting new therapies reviewed in this issue is built.
The discovery of heparin: who did what when?
Heparin is a highly sulfated, heterogeneous mixture of glycosaminoglycans of varying size and biologic activity extracted from animal tissues: liver, muscle, beef lung, and more recently, porcine intestinal mucosa. We now understand that heparin exerts its antithrombotic activity by binding to and enhancing the activity of the natural anticoagulant protein antithrombin 3. It functions in a catalytic manner, recycling from coagulation serine protease-antithrombin complexes to native antithrombin more than 30,000 times each second.
The discovery of heparin is a fascinating piece of medical history. James McLean was an ambitious medical student from California who matriculated at Johns Hopkins with the goal of becoming an academic surgeon. In 1916, he obtained a position in the laboratory of Dr. William Howells, a pre-eminent Hopkins physiologist. He was assigned the task of purifying cephalin or thromboplastin, a tissue-derived or blood cell–derived phosphatide thought to initiate coagulation. His goal was to ascertain the purity of the laboratory’s cephalin preparation, derived from dog liver, and confirm that its procoagulant activity resided in the purified phosphatide fraction and was not due to a contaminant. Unexpectedly, he found that certain phosphatide fractions separate from cephalin itself had anticoagulant properties. He published a paper on his finding in 1917 and demonstrated its anticoagulant property by injecting the heparphosphatide into living dogs.
McLean was awarded a fellowship to work at University of Pennsylvania in 1917 and did not continue work on the heparphosphatide there. He received a master’s degree for his work on cephalin at Penn and was credited by Johns Hopkins for his second year of medical school. He then volunteered for the American Ambulance Corps and, in 1917, worked in France caring for wounded soldiers. In the fall of 1917, he returned to Johns Hopkins to finish his medical education. He also returned to Howell’s laboratory but worked exclusively on cephalin, trying to develop ways to control surgical bleeding with cephalin-coated gauze. Howell, in his 1917 Harvey Lecture, credited McLean with the initial discovery of an anticoagulant phosphatide in liver. McLean had hoped to return to Howell’s laboratory between his third of fourth year of medical school to resume work on the heparphosphatide, but this never materialized.
Another medical student, Emmett Holt, assisted Howell and isolated another compound from liver with anticoagulant properties, which they termed heparin to distinguish it from the heparphosphatide of McLean. Although McLean focused on cephalin and its applications, Howell continued to study anticoagulants from liver and, in 1922, presented a paper to the American Physiologic Society on the isolation of an anticoagulant molecule from an aqueous extract of liver, which did not contain phosphate but was rich in carbohydrate, particularly glucuronic acid. This material is most likely the compound we recognize today as heparin. The potential clinical importance of such an anticoagulant was quickly recognized and heparin attracted the attention of Nobel Laureate Charles Best and his colleagues at the Connaught Laboratories in Toronto. Best assembled a team of chemists, physiologists, and clinicians with the goal of establishing heparin as a useful drug. A Swedish group also began work on heparin with similar goals. By the 1940s, heparin was ready for clinical use. However, its debut was delayed until the end of the Second World War.
While the heparin project evolved in Canada and Sweden, McLean worked briefly at several academic institutions trying to establish himself as an academic physician. He eventually settled in Columbus Ohio in a private practice and worked in a local hospital. At this time, he initiated a long campaign to establish himself as the discoverer of heparin. He corresponded with Best and others on this subject and tried to argue that the material he isolated in 1916 was identical to the heparin later isolated by Holt and Howell, and further purified by Best and his colleagues. McLean wrote to many of the leaders of academic medicine and science about his role in the discovery of heparin and began a detailed account, which he never finished because of his death in 1957. Two years after his death, his unfinished commentary “The Discovery of Heparin” was published in Circulation .
After the fact, it is hard to assess the 2 conflicting tales. It is certain that McLean found something in dog liver that acted as an anticoagulant and I think he deserves credit for the concept of a natural anticoagulant. Howell and others are probably also correct that the material eventually developed into a clinically useful anticoagulant differed from the material McLean originally discovered in the Howell laboratory. Most people, who have not delved into all the details, believe McLean discovered heparin and did not receive appropriate credit. In fact, I have taught this to my students. I now think that McLean was over-reaching by demanding sole credit for the discovery. Had he been willing to share the credit with his mentor Howell and, perhaps, others in the laboratory who worked on the project, he might have died a happier man. We will never know.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree