Category
Adults (20+ years)
Youth (2–19 years) CDC, AAP, IOM, ES, IOTF
Underweight
BMI < 18.5
BMI < 5th percentile for age
Normal weight
BMI 18.5–24.9
BMI ≥ 5th to < 85th percentile
Overweight
BMI 25–29.9
BMI ≥ 85th to < 95th percentile
Obesity
BMI ≥ 30
BMI ≥ 95th percentile
Class III obesity (super obesity)
BMI ≥ 40
Not useda
In children , defining criteria for obesity is more complex, as the age, sex, expected growth curves, and body composition must all be factored in. A child’s weight status is determined using an age- and sex-specific percentile for BMI rather than the BMI categories. The Centers for Disease Control growth charts are used to determine the corresponding BMI-for-age and sex percentile. For children and adolescents (aged 2–19 years), overweight is defined as a BMI at or above the 85th percentile and lower than the 95th percentile for children of the same age and sex, while obesity is defined as a BMI at or above the 95th percentile for children of the same age and sex [15]. Figure 2.1 shows the classification of obesity for children based on the American Academy of Pediatrics, the Institute of Medicine, the Centers for Disease Control, and the International Obesity Task Force.
The Economic Burden of Obesity
Globally, in an analysis of 199 countries, 1.46 billion adults worldwide were estimated as overweight, with 502 million estimated as obese [3]. The global economic burden of obesity accounts for an average of 0.7–2.8 % of a country’s total health-care costs [16]. These costs represent the monetary value of health-care resources devoted to managing obesity-related disorders. This includes such costs as those incurred through the use of outpatient clinics and visits, hospitalizations, pharmaceutical therapy, laboratory testing, and chronic care. Obese individuals have, on average worldwide, medical costs 30 % higher than those with normal weight [16]. Interestingly, in the USA specifically, the medical economic burden of obesity is higher: an estimated US$ 75 billion in 2003 [17], accounting for 4–7 % of total health-care expenditure. The increase in costs seen in obese individuals tends to be largely driven by the increased incidence of type 2 diabetes, the increased cardiovascular burden, and obesity-related cancers [18].
Overweight/obesity in middle age appears to have long-term adverse consequences for health-care costs as one ages. A review of US Medicare data collected from 1984 to 2002 showed that after multivariate analysis, Medicare health charges were significantly higher by baseline BMI in both men and women [19]. This held true for overall costs, and costs specifically related to diabetes and cardiovascular disease. After adjusting for variables such as baseline age, race, education, and smoking, the total average annual medical-related charges for overweight , obese, and severely obese men were US$ 8390, $ 10,128, and $ 13,674, respectively. This is a significant trend over normal-weight men who, as a group, averaged an annual health-care cost of $ 7205. Other US data show that compared to normal-weight individuals, obese patients incur 27 % more outpatient visits, and 80 % more prescription costs [20]. In addition, in the inpatient setting, obese patients have an increased cost of 46 % over nonobese patients. Similar trends have been reported in the UK, France, and the Netherlands.
The Health Burden of Obesity
The World Health Organization describes obesity as one of the most neglected public health problems we face today [21]. The health implications of obesity are not geographically limited. Sequelae of obesity include commonly thought of conditions, such as hypertension, heart disease , fatty liver, and diabetes, [22] to more esoteric associations such as infertility [22], idiopathic intracranial hypertension [23], and gout [24]. The incidences of certain cancers also increase with obesity, including cancers of the breast, ovaries, esophagus, colon, liver, pancreas, endometrium, and prostate [25].
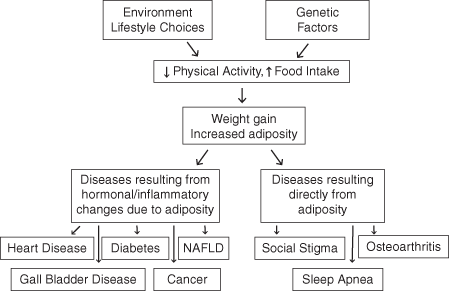
The health conditions associated with obesity are thought to arise as either a direct consequence of adiposity—such as with social stigmatization, sleep apnea, and osteoarthritis; or via the various changes associated with the increase in adipose cell hypertrophy and/or hyperplasia (Fig. 2.1) [26]. It is important to remember that adipose tissue is a functional endocrine organ, with secretory products such as cytokines (interleukin (IL)-1 and 6) and tumor necrosis factor alpha (TNF-α). These cytokines have further effects, including suppression of adiponectin—which worsens insulin resistance. Diabetes , cancer, cardiovascular disease, and non-alcoholic liver disease are a few examples of disease states attributed in part to these hormonal and metabolic alterations. Having abdominal obesity seems to worsen these associated conditions, in part because of the high influx of free fatty acids, adipokines, and cytokines into the portal circulation by virtue of approximation. Subsequent hepatic production of very-low-density lipoprotein (VLDL) and dysregulation of insulin release set off a cascade of metabolic derangements [22].
Social Stigmatization
Many societies tend to chastise those who are overweight, and many consider those with weight issues as being unable or unwilling to control impulsive/compulsive behaviors. There is often public disapproval expressed openly by colleagues, neighbors, family members, and acquaintances. Such reproach often results in measurable changes in the quality of life parameters reported by obese subjects [26, 27]. These changes are more profound in women, and tend to reverse with intentional weight loss [28, 29]. Children and adolescents also tend to suffer the psychosocial consequences of obesity, including alienation [30], distorted peer relationships, poor self-esteem [31, 32], anxiety [33], and depression [34, 35]. The risk of psychosocial morbidity increases with increasing age during childhood, and is greater among girls than boys [36–38].
The distorted and negative self-images that develop in adolescence often persist into adulthood, especially in women. Data from the National Longitudinal Survey of Youth indicate that women who were obese in late adolescence and early adulthood completed fewer years of advanced education, and had lower rates of marriage and higher rates of poverty compared to their non-obese peers [39]. Interestingly, these long-term social repercussions were not nearly as profound in obese men.
Sleep Apnea
In the absence of underlying pulmonary disease, obese patients are noted as having pulmonary-related issues only in the presence of significant obesity. The main obesity-related change in pulmonary function testing is an increase in residual lung volume associated with an increase in intra-abdominal pressure [40, 41]. While these pulmonary function changes may be mild, the other effects of obesity on the respiratory system can be quite significant. Obstructive sleep apnea (OSA) is a syndrome characterized by episodic hypopnea or apnea due to recurrent partial or complete upper airway obstruction during sleep. Obesity is the most documented risk factor for OSA. Significant sleep apnea is present in approximately 40 % of obese individuals, and the prevalence of OSA progressively increases as the BMI increases [42].
OSA frequently coexists with, and may be one of the causes of obesity hypoventilation syndrome (OHS). OHS is defined as obesity and chronic alveolar hypoventilation (arterial carbon dioxide tension [PaCO2] > 45 mmHg) during wakefulness, which occur in the absence of other conditions that cause hypoventilation [43].
Osteoarthritis
Diseases of the bone including osteoarthritis and other joint issues are directly related to the weight placed on the joints by obesity [44]. For example, the incidence of knee osteoarthritis was found to be increased in men in heaviest quintile of weight compared with those in the lightest three quintiles (age-adjusted relative risk, 1.51; 95 % confidence interval (CI), 1.14–1.98), and was further increased in women in the heaviest quintile versus those in the lightest three quintiles (relative risk 2.07; 95 % CI, 1.67–2.55) [44]. There is some suggestion that non-weight-bearing joints also suffer changes in the obese; however, the mechanism underlying these changes is not known.
Nonalcoholic Fatty Liver Disease (NAFLD)
NAFLD is a term describing a collection of liver abnormalities including hepatomegaly, elevated liver enzymes, and changes in histology which include (in progressive order) steatosis, non-alcoholic steatohepatitis, fibrosis, and cirrhosis [45]. Once NAFLD has progressed to cirrhosis, liver failure may ensue. Obesity is associated with this clinical spectrum of liver damage and disease [45, 46]. The pathogenesis of NAFLD in overweight and obese individuals is not fully understood, but insulin resistance appears to be an important component [47]. A retrospective analysis of liver biopsies in individuals who were overweight and obese without any other underlying contributors to liver disease showed the presence of fibrosis in 30 % of samples, and cirrhosis in a further 10 % [48]. Other authors have performed cross-sectional analysis of liver biopsies and suggest that the prevalence of steatosis is 75 % in the obese population [49]. In another study, metabolic syndrome (of which obesity and insulin resistance are components) was associated with an odds ratio (OR) of 3.5 for the development of severe liver fibrosis [50].
Hypertension
Obesity is associated with hypertension. The relation between obesity and hypertension is clinically important because weight loss may lead to a significant fall in systemic blood pressure (BP) [51, 52]. The impact of obesity on the presence of hypertension may have ethnic differences. It is estimated that weight control would eliminate hypertension in 28 % of the Black population. This is almost doubled to an estimated 48 % in the White population [53].
The mechanism by which obesity raises the BP is not well understood. One postulate is that hyperinsulinemia is the cornerstone of this relationship [54], and many mechanisms have been proposed to explain the resultant increase in BP including increased sympathetic activity [55], volume expansion due to increased renal sodium reabsorption [56], endothelial dysfunction [57], upregulation of angiotensin II receptors [57], and decreased cardiac natriuretic peptide [58]. The risk of hypertension appears to be greatest in people who have predominantly upper body and abdominal obesity. The mechanism by which upper body obesity raises BP remains unclear. Insulin resistance is thought to be a central component, leading to impaired glucose tolerance and hyperinsulinemia. Hyperinsulinemia may then raise the BP by the mechanisms noted above. Despite these observations, insulin resistance or hyperinsulinemia as a cause of hypertension remains controversial. There is also mounting evidence that leptin may have a role in obesity-related hypertension, via increased sympathetic activity [54].
The sleep apnea syndrome associated with obesity is an additional contributing factor to the development of hypertension [59]. It is thought that activation of the sympathetic nervous system, elevated aldosterone levels, and increased levels of endothelin by repeated episodes of hypoxia are responsible for the associated hypertension [60].
The presence of sustained weight loss has a beneficial effect on hypertension. The long-term effect of weight loss was evaluated over an 8-year period among overweight 30- to 49-year-olds and overweight 50- to 65-year-olds [61]. A sustained reduction in weight of 6.8 kg or more was associated with a 22 % reduction in relative risk for developing hypertension (defined by 140/90 mmHg) in the younger age group and 26 % reduction in relative risk in the older overweight population. A simple relationship to remember is that for each 1 kg of weight loss, systolic and diastolic pressures fall by approximately 1 mmHg [62].
Cardiovascular Disease and Stroke
Overweight and obesity are associated with multiple cardiovascular abnormalities. In addition to an association with coronary artery disease, there is an increase in cardiac volume, cardiac work increases, and this may produce cardiomyopathy and heart failure.
Heart Failure
It is often forgotten that obesity can be an independent etiology of heart failure that is just as significant as hypertension, coronary disease, and diabetes. Evidence from the Framingham Heart Study showed that obesity doubled the risk of heart failure. In the 6000 subjects studied, multivariate analysis showed a 5–7 % increase in risk for every 1 kg/m2 increase in BMI [63]. The physiologic processes responsible for this increase are likely multifactorial, and include an increase in cardiac work, an association with insulin resistance, subclinical right ventricular dysfunction, and association with diabetes, sleep apnea, and hypertension.
Cardiac Rhythm Abnormalities
Patients with a BMI of greater than 30 kg/m2 are significantly more likely to develop atrial fibrillation than individuals of normal weight. [64]. This increased risk has also been shown in many studies, and appears to be particularly associated with sustained atrial fibrillation as compared to transient or intermittent atrial fibrillation [65]. There does not appear to be an increased risk in ventricular dysrhythmias associated directly with increasing BMI or weight gain.
Coronary Heart Disease (CHD)
The Nurses’ Health Study has shown a 3.3-fold higher risk of developing coronary artery disease in women with a BMI greater than 29 when compared to lean women [66]. When followed longitudinally, there is also an associated increase in heart disease with weight gain in women over time. This finding was highest in women who gained over 20 kg, and was independent of starting BMI. The association between obesity and CHD has also been observed in many other large-scale population-based studies [67–69]. The distribution of body fat again appears to play a role, with those subjects having predominantly abdominal or central fat being the group at greatest risk. Using the waist-to-hip ratio as a measurement for abdominal obesity in a female cohort, researchers have shown that a value of > 0.88 provides a threefold higher risk of CHD when compared to women with a ratio of < 0.72 [70]. Others have shown that the risk appears to increase sharply once the ratio is > 0.8 [71].
It is well known that dyslipidemia is an important risk factor for the development of atherosclerosis. The classic dyslipidemic pattern of obesity consists of an elevated triglyceride (TG) level and a decreased level of high-density lipoprotein (HDL). While the decrease in HDL may be an important contributor to the development of heart disease in obesity, perhaps more suspect is the changes associated with the character and quality of low-density lipoprotein (LDL) seen in obesity. Central fat distribution is associated with an increase in small, dense LDL. This form of LDL is more atherogenic than the alternate large fluffy LDL [72]. It has also been postulated that obesity poses an increased CHD risk because of associated low concentrations of adiponectin, which has antiathrogenic properties and lowers insulin resistance [73].
Stroke
The data linking obesity to stroke risk are not as clear as the data linking obesity to CHD. The Emerging Risk Factors Collaboration reviewed data on over 85,000 subjects and found that the risk of ischemic stroke increased by 20 % for every 1 standard deviation increase in BMI [74]. However, this risk was dramatically attenuated once adjusted for age, smoking, hypertension, diabetes, and cholesterol status. Some studies have shown an increased risk of both ischemic and hemorrhagic stroke in obese patients [75]. Most other studies have not seen this association with hemorrhagic stroke [76]. The Nurses’ Health Study indicates that both a BMI of greater than 27 kg/m2 and accelerated weight gain after age 18 are associated with increased ischemic stroke risk. The relative risk reported was 2.4 for a BMI of 32 kg/m2 or greater when compared to a BMI of 21 kg/m2 or less [77]. The Women’s Health Study also reported similar findings [78].
Insulin Resistance and Diabetes
Insulin Resistance
Insulin resistance and type 2 diabetes are significant health risks well known to be associated with obesity , such that even mild detriment to insulin release has been shown to have profound effects on metabolic processes, and thus regulation of weight and obesity [79]. Insulin resistance is stimulated by fat deposited within cells and cytokines (IL1, IL6, TNF-α) secreted by adipocytes that actively suppresses insulin sensitizers. Insulin resistance is only one part of the pathophysiology of type 2 diabetes, with B cell dysfunction in the pancreas also playing a role. Notably, the connection between insulin resistance and inflammatory pathways provides an explanation for the comorbid association between type 2 diabetes and obesity, examined further in clinical studies associating weight loss with an increase in insulin sensitivity in adults (P< 0.002) [80, 81]. Environmental, genetic, and societal factors contribute to the development and repercussions of obesity and insulin resistance, as well as differences in ethnicity and gender. Men and African Americans exhibit a greater prevalence for insulin resistance, with African Americans constituting the highest rate of diagnosed diabetes among all the races at 11.2 % [82].
Diabetes
Data from the Behavioral Risk Factor Surveillance System (BRFSS) from 2001 of 195,005 adults in the USA showed that obese adults (BMI ≥ 40) have greater than a sevenfold OR for a diagnosis of diabetes than the average adult [82]. This figure may be a staggering underestimation of the true presence of diabetes in the population due to various survey constraints within the survey population and the criterion that only doctor-diagnosed diabetes was tabulated, though an estimated 27 % of those affected by diabetes remain undiagnosed [83]. The link is irrefutable when the converse association is considered: 64 % of men and 77 % of women with type 2 diabetes are overweight or obese. There is also sufficient evidence linking obesity to the development of gestational diabetes mellitus. Using a regression analysis between prepregnancy BMI and presence of gestational diabetes, researchers calculated the percentage of gestational diabetes attributed to obesity and found a statistically significant higher risk of gestational diabetes correlated to higher BMI and 46.2 % of gestational diabetes occurrences ascribed to being overweight , obese, or extremely obese (95 % CI = 26.1, 56.3) [84]. With an estimated $ 174 billion spent annually on the treatment of diabetes and a projected number of one in three Americans with diabetes by 2050, the health burden of obesity and its connection to insulin resistance and type 2 diabetes poses as an immense public health problem for worldwide populations [85] .
Cancers
In 2008, there were an estimated 12.7 million cancer cases and 7.6 million cancer deaths worldwide [86, 87]. Together, modifiable risks such as tobacco use, excess weight, poor diet, and inactivity are thought to account for almost 70 % of all cancers in the USA [88]. Obesity as a sole risk factor is estimated to cause 20 % of all cancers [89]. Excess weight and obesity are associated with an increased risk of developing multiple cancers including colorectal, postmenopausal breast, endometrial, renal, and esophageal cancer. The attributable risk of excess weight ranges from 9 % (postmenopausal breast cancer) to 39 % (endometrial cancer) [90]. Newer data suggest that excess body weight and increased body fat also have a direct association with additional cancers including pancreas, thyroid, non-Hodgkin lymphoma, leukemia, and myeloma [91].
Weight gain itself is also associated with cancer risk. For example in a Canadian report, men who gained ≥ 21 kg after age 20 had a 60 % higher risk of colorectal cancer than men who gained only 1–5 kg [92]. In another study, women who lost ≥ 10 kg after menopause and kept it off saw a 50 % reduction in breast cancer risk [93].
The exact mechanism behind the association of weight with cancer development is not clear—and is likely multiple. One contributing factor is thought to be related to the increased aromatization that occurs in fat tissue, resulting in higher levels of estrogen. This may be a factor in endometrial cancer and breast cancer risk. Other proposed mechanisms include the influence of obesity and weight gain on insulin resistance and subsequent effects on inflammation. The latter may be particularly important in colon cancer [89].
A recent report suggests that bariatric surgery is associated with a 60 % reduction in overall cancer mortality (5.5 vs. 13.3 per 10,000 person-years). The follow-up for this study was 7 years, however, more data of this sort are needed to confirm this observation [94]. In addition, this benefit seen with bariatric surgery may not be the case with every cancer (see colon cancer).
While the above refers directly to excess body weight as a contributor to cancer risk, it is important to remember that physical inactivity and poor dietary intake are also contributors to cancer risk. While often intertwined with obesity, these two factors are independent and carry with them their own cancer risks that are beyond the scope of this chapter’s discussion.
What about the role of weight after the diagnosis of cancer is made? The relative impact of weight on the prognosis and recurrence rates of cancer is dependent on the type of cancer being discussed.
Breast Cancer
Women who are obese at the time of breast cancer diagnosis have a 30 % higher risk of breast-cancer-related mortality as compared to leaner women [95]. The reasons for this remain unclear and causality versus association remains debated. The authors who reported this finding also noted that the association holds true in both premenopausal and postmenopausal women, with the relative risk of death from breast cancer in obese versus nonobese individuals being 1.47 in premenopausal and 1.22 in postmenopausal women, respectively. It has also been reported that women with a BMI of greater than 35 kg/m2 have a 60 % higher risk of breast cancer recurrence as compared to women with BMIs of less than 25 kg/m2 [96, 97].
In addition to weight at the time of diagnosis, weight gain after the diagnosis of breast cancer may also be associated with an increased risk of recurrence, although the data are inconsistent. In the Nurses’ Health Study, women previously treated for breast cancer who gained 0.5–2 kg/m2 and those who gained more than 2 kg/m2 had risks of breast cancer death of 35–64 % compared to women who maintained a stable weight [98]. However, other analyses have not supported these findings [99].
There are relatively few studies evaluating the efficacy and potential benefits of weight loss interventions in breast cancer survivors. The largest weight loss study to date in breast cancer survivors has been the Lifestyle Intervention Study for Adjuvant Treatment of Early Breast Cancer (LISA) [100]. In this study, more than 300 postmenopausal women with hormone receptor-positive breast cancer were randomly assigned to a weight loss intervention arm (including counseling by regular phone calls) or to usual care. The authors found that women randomized to the intervention lost approximately 4.5 kg more than the control. More importantly, there were significant improvements in physical functioning scores in those who lost weight. There is also evidence to suggest that the incidence of breast cancer may be decreased in women following bariatric surgery—although the results did not reach significance, a large-scale study in Utah found a lower incidence of breast cancer in gastric bypass patients compared with severely obese controls (hazard ratio (HR) of 0.91; 95 % CI, 0.67–1.24; P = 0.54) [101]. Other studies have also suggested that cancer rates are reduced following bariatric surgery, particularly in women, although the group sizes in most cases prevented statistical analysis of site-specific cancers [102–105].
Prostate Cancer
Obesity is associated with worse outcomes among men with prostate cancer. However, whether changes in weight following a diagnosis of prostate cancer can modify prognosis is currently unknown. A study of almost 2000 men undergoing prostate biopsy showed that the risk of a high-grade prostate cancer (i.e., Gleason score ≥ 7) increased with an increasing BMI [106]. Similarly, there appears to be an association with obesity and advanced disease. A 2012 meta-analysis demonstrated a relative risk increase of 9 % for each 5 kg/m2 increase, and an inverse relationship between BMI and the development of localized prostate cancer [107]. Prostate-specific mortality appears to increase by 20 % for each 5 kg/m2 increase in BMI [108].
Colorectal Cancer
Studies have shown that patients diagnosed with nonmetastatic colorectal cancer with a high pre-diagnosis BMI (BMI ≥ 30 kg/m2) have significantly poorer cancer-specific survival when compared to those within the normal BMI range [109]. The authors of this study did not see an association with post-diagnosis BMI and outcomes . In contrast, another study demonstrated that stage II and III colon cancer patients with BMI > 35 kg/m2 after surgery had less disease-free survival compared to normal-weight patients [110]. There are little data on weight loss and survival benefit in patients with colon cancer.
Endometrial Cancer
Unlike prostate cancer, there appears to be an association of a less aggressive type of endometrial cancer with obesity [111]. It is thought that there is a greater likelihood of developing an estrogen-responsive tumor in women with a higher level of circulating estrogen. Accordingly, severely obese women were also more likely to present with stage I disease (77 vs. 61 %) or low-grade tumors (44 vs. 24 %). Despite this, among women with endometrial carcinoma, obesity is associated with an increased risk of death [112]. The risk of dying from endometrial carcinoma among those with the highest BMI (≥ 40 kg/m2) was 6.25-fold higher than that of normal-weight women [25]. Unfortunately, the benefits of weight loss on outcome and recurrence are not well studied in endometrial cancer.
Reproductive Issues
Obesity affects ovulation, response to fertility treatment, pregnancy rates, and outcome. The National Health and Nutrition Examination Survey found that 32 % of women between the ages of 20 and 39 were obese [113]. The prevalence varied with ethnicity, and was the highest (56 %) in non-Hispanic blacks. Adipose tissue is an endocrine organ, and women with obesity have elevations in leptin and reductions in adiponectin, both of which may lead to insulin resistance. Obesity can also be associated with changes in estrogen and androgen levels. All of these factors can impact fertility.
Polycystic ovary syndrome (PCOS) is the most common reproductive disorder in women. PCOS is associated with obesity and ovulatory dysfunction along with hyperandrogenemia and insulin resistance. Restoration of ovulation often occurs with weight loss. One study reports that after a weight loss ranging from 4.8 to 15.2 kg (mean 9.7 kg), significant reductions in the concentration of luteinizing hormone (LH), fasting insulin, and testosterone were noted, and most of the women ovulated after weight reduction [114]. Similar results have been reported by other researchers [115].
In women without a cause of ovulatory dysfunction, obesity is associated with a decrease in spontaneous pregnancies and also with an increased length of time to achieving conception [116–118]. Obesity appears to be associated with subfertility and poor reproductive outcome regardless of the mode of conception, but the exact physiological mechanisms linking obesity to decreased fertility are not known. While some studies have shown weight loss results in higher live birthrates [119, 120], other authors have not shown this benefit in outcome. Large-scale studies have yet to be performed.
In women undergoing fertility treatment, some studies have shown that obesity is associated with insufficient follicular development, and lower oocyte counts during treatment [121–123]. Other studies have shown that ovulation-inducing medical regimens must be given in higher doses to allow for success of treatment in the obese female patients [124, 125]. In a recent meta-analysis including 33 studies and almost 48,000 in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) treatment cycles, women who were overweight or obese had reductions in clinical pregnancy rates and live birthrates that were marginal but significant when compared to normal-weight women. Overweight and obese women in this analysis also had significantly higher miscarriage rates (RR = 1.31) than normal-weight women [126].
While the data appear compelling in associating obesity with suboptimal fertility, many questions remain. Much of the literature thus far has been suboptimal, with poor patient selection for many trials and lack of viable controls. In addition, many of the studies conducted have been retrospective in nature. Thus, while an association can be seen, there is no clear conclusion or consensus on the mechanism of obesity on fertility.
Life Expectancy
While in the past two centuries life expectancy has lengthened due to factors such as lifesaving scientific discoveries, medical progress, and enhanced hygiene, the negative impact of obesity may end this trend. As a result, the current generation may be the first in evolution which has a lower life expectancy than their parents [127]. An analysis of data from the Framingham study confirmed that obesity is related to a reduction in life expectancy—for subjects who were 40 years old, obese men and women both had reductions in life expectancy of 6–7 years when compared to non-overweight cohorts [128]. The Prospective Studies Collaboration analysis [129] found that each 5 kg/m2 incremental increase in BMI over 25 is associated with increased risks for CHD (39 %), stroke (39 %), diabetes (216 %), cancer (10 %), and respiratory disease (20 %).
Effect of Fitness
Fitness level is also an important factor in obese individuals. In one study, higher levels of fitness appeared to negate some of the excess cardiovascular mortality risk associated with obesity in men [130]. In contrast, in the Lipids Research Clinics and the Nurses’ Health Studies, both physical fitness and adiposity were independent predictors of mortality, and higher levels of fitness did not negate the association between obesity and mortality [131, 132]. Recently, there has been a considerable debate as to whether or not it is possible to be “fat but fit,” with earlier studies seeming to suggest that individuals who were overweight or even obese but who were also physically fit and metabolically healthy had no greater risk of mortality as a result from heart disease or cancer than their normal-weight counterparts [133–135]. However, a recent meta-analysis has challenged these findings, indicating that metabolically healthy obese individuals had increased risk for all-cause mortality and/or cardiovascular events (relative risk (RR), 1.24; 95 % CI, 1.02–1.55) when compared to metabolically healthy normal-weight individuals, and also found that all metabolically unhealthy groups (normal weight, overweight, and obese) exhibited similarly increased risks [136].
The Burden of Obesity in Children
Disease Burdens on Obese Children
The prevalence of obesity has increased dramatically across all countries, races, and social factors, with an estimated global prevalence of childhood obesity reaching 170 million children under the age of 18, such that life expectancy for younger generations is projected to be shorter for the first time in the modern era [127, 137] (see Life Expectancy). The childhood obesity epidemic is the result of a culmination of factors that include biological factors such as genetic factors and family histories of obesity, diabetes mellitus and hypertension, social determinants, and the newer factor of technological advancements that are associated with obesogenic behaviors and which promote sedentary lifestyles [138, 139]. Comorbidities significantly associated with childhood obesity include diabetes , sleep apnea, fatty liver disease, and cardiovascular disease [140]. Childhood obesity also adversely affects lipid profiles [141], and is associated with elevated systolic and diastolic BP, high risks of hypertension, and adverse total cholesterol to HDL ratios, which can persist throughout childhood and adolescence and into adulthood [142–144]. Childhood obesity has also been shown to increase the risk for menstrual problems later in life—for example, one study found that obesity at 7 years of age was associated with an increased risk of menstrual problems by age 33 (OR = 1.59) [145].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree