This article describes the current role and perspectives of “new” allogeneic stem cell transplantation (alloSCT) in the treatment of chronic lymphocytic leukemia (CLL). Clinical trials and minimal residual disease (MRD) studies provide clear evidence that graft-versus-leukemia activity is working in CLL and can provide long-term MRD-free survival in up to 50% of patients undergoing alloSCT. AlloSCT is effective also in poor-risk CLL as defined by the European Group for Blood and Marrow Transplantation transplant consensus. Novel forms of (reduced intensity) conditioning have resulted in dramatic reduction of early morbidity and mortality of alloSCT in CLL.
Key points
- •
Novel forms of (reduced intensity) conditioning have resulted in dramatic reduction of early morbidity and mortality of allogeneic stem cell transplantation (alloSCT), making this procedure now suitable for comorbid and elderly patients.
- •
This “new” alloSCT is working particularly well in chronic lymphocytic leukemia (CLL) based on strong graft-versus-leukemia efficacy.
- •
New alloSCT is effective also in poor-risk CLL and can provide long-term disease-free survival.
- •
Preliminary evidence suggests that alloSCT indeed can change the natural history of poor-risk CLL, and novel CLL-targeting drugs may have the potential to further improve transplant outcome.
To improve the prognosis of patients with poor-risk chronic lymphocytic leukemia (CLL), efforts to develop effective treatment strategies have focused on autologous and allogeneic stem cell transplantation (SCT) more than 20 years ago. The rationale for this was the assumption that intensive myeloablative treatment might be able to eradicate the leukemic clones, thereby curing the disease. However, myeloablative treatment is associated with profound nonhematopoietic toxicity, making this procedure often intolerable for elderly and comorbid individuals who represent most patients with CLL. Moreover, recent mature results from studies on autologous stem cell transplantation (autoSCT) and T-cell depleted allogeneic stem cell transplantation (alloSCT) have suggested that myeloablative therapy alone is generally not sufficient to cure CLL.
After it had been recognized that in CLL the antileukemic effect of alloSCT is largely, if not entirely, due to the graft-versus-leukemia (GVL) activity conferred with the hematopoietic stem cell graft and that engraftment and GVL activity can be achieved without preceding myeloablative treatment, the avenues for a completely new form of allogeneic transplant were opened. This “new” alloSCT is fundamentally different from the traditional myeloablative transplant, is applicable to a large proportion of the CLL target population, and represents the most effective and only curative treatment of CLL available today. Its clinical effectiveness relies on the initiation of cellular immune therapy permanently active in the patient, thereby providing a treatment modality that is in a biologic sense completely different from any other cytotoxic or immunologic therapy. Accordingly, the numbers of (nonmyeloablative) allotransplants for CLL are steadily increasing, making CLL now the most frequent indication for alloSCT among all lymphomas in the European Group for Blood and Marrow Transplantation (EBMT) registry. In contrast, autoSCT is rapidly declining ( Fig. 1 ).
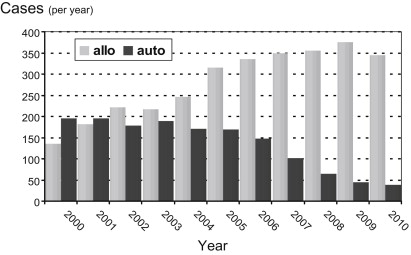
This overview summarizes the knowledge characterizing the efficacy and tolerability of modern alloSCT strategies in CLL and describes the resulting role of alloSCT in the current therapeutic arsenal for CLL.
Evidence for efficacy of new alloSCT in CLL
The basis of new (nonmyeloablative or reduced-intensity conditioning [RIC]) alloSCT in CLL is that GVL effects are active. Evidence for GVL efficacy in CLL derives from the observation that, in contrast to autoSCT or other intensive therapies, the relapse incidence seems to decrease over time after RIC alloSCT. Accordingly, all larger studies on RIC alloSCT in CLL show a plateau at 40% to 50% in the disease-free survival curve ( Table 1 ). Furthermore, GVL activity in CLL is indicated by a reduced relapse risk in the presence of chronic graft-versus-host disease (GVHD), and an increased relapse risk associated with the use of T-cell depletion.
| Dreger et al, 2010 | Sorror et al, 2008 | Brown et al, 2012 | Khouri et al, 2011 | Michallet et al, 2011 | |
|---|---|---|---|---|---|
| N | 90 | 82 | 76 | 86 | 40 |
| Conditioning regimen | Nonmyeloablative (fludarabine-cyclophosphamide ± antithymocyte globulin) | Nonmyeloablative (fludarabine—low-dose total body irradiation) | Reduced intensity (fludarabine-busulfan) | Nonmyeloablative (fludarabine-cyclophosphamide-rituximab) | Nonmyeloablative (fludarabine-cyclophosphamide-rituximab) |
| Alternative donors a (%) | 59 | 37 | 63 | 50 | 0 |
| Relapse incidence (%) | 40 (4 y) | 38 (5 y) | 40 (5 y) | nr | 22 (3 y) |
| Progression-free survival (%) | 42 (4 y) | 39 (5 y) | 43 (5 y) | 36 (5 y) | 46 (3 y) |
| Overall survival (%) | 70 (4 y) | 50 (5 y) | 63 (5 y) | 51 (5 y) | 55 (3 y) |
| Follow-up (y) | 3.8 (0.6–8.5) | 5 (0.9–7.3) | 5.1 | 3.1 (0.9–10.9) | 2.3 (0.3–5.9) |
The most convincing proof of the GVL principle in CLL comes from studies analyzing the kinetics of minimal residual disease (MRD) after RIC alloSCT. MRD denotes a disease burden remaining after specific therapy, which is detectable only at a subclinical level. For CLL, this is defined as a contamination of 5 CLL cells or less per nanoliter of peripheral blood in the absence of clinical signs or symptoms of the disease. Patients showing less than 1 CLL cell in 10,000 benign leukocytes in peripheral blood or bone marrow are considered as being MRD negative.
In CLL, MRD can be quantified even at low levels by sensitive (>10E4) polymerase chain reaction– or flow cytometry–based assays. We analyzed MRD kinetics in 43 patients who had undergone RIC alloSCT within the prospective CLL3X trial of the German CLL Study Group (GCLLSG) or a pilot study and remained event-free 1 year after alloSCT. Of these, 32 patients had become MRD negative at 1 year after transplant, while in 11 patients the MRD level did not decrease below the threshold of detection until this landmark. Achievement of MRD negativity was clearly linked to immune intervention, such as tapering of immunosuppression or donor lymphocyte infusions ( Fig. 2 ). Absence of detectable MRD 1 year after alloSCT was strongly associated with a reduced risk of clinical relapse (hazard ratio [HR], 0.037; 95% confidence interval [CI], 0.008–0.18; P <.0001). Similar observations were reported by other investigators.
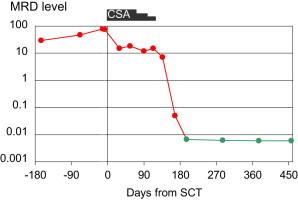
Altogether, MRD kinetics studies consistently indicate that permanent MRD negativity after alloSCT for CLL can be reached in the context of immunomodulating intervention. Both the durability of MRD remission and its sensitivity to immunomodulation strongly suggest that GVL activity is effective in CLL. GVL activity in CLL seems to be closely correlated to chronic GVHD, implying that it essentially depends on allogeneic effects with broader specificity rather than on a CLL-specific reactivity of donor GVL effector cells.
In summary, clinical trials and MRD studies provide clear evidence that GVL is working in CLL and can provide long-term MRD-free survival in up to 50% of patients undergoing RIC alloSCT.
Evidence for efficacy of new alloSCT in CLL
The basis of new (nonmyeloablative or reduced-intensity conditioning [RIC]) alloSCT in CLL is that GVL effects are active. Evidence for GVL efficacy in CLL derives from the observation that, in contrast to autoSCT or other intensive therapies, the relapse incidence seems to decrease over time after RIC alloSCT. Accordingly, all larger studies on RIC alloSCT in CLL show a plateau at 40% to 50% in the disease-free survival curve ( Table 1 ). Furthermore, GVL activity in CLL is indicated by a reduced relapse risk in the presence of chronic graft-versus-host disease (GVHD), and an increased relapse risk associated with the use of T-cell depletion.
| Dreger et al, 2010 | Sorror et al, 2008 | Brown et al, 2012 | Khouri et al, 2011 | Michallet et al, 2011 | |
|---|---|---|---|---|---|
| N | 90 | 82 | 76 | 86 | 40 |
| Conditioning regimen | Nonmyeloablative (fludarabine-cyclophosphamide ± antithymocyte globulin) | Nonmyeloablative (fludarabine—low-dose total body irradiation) | Reduced intensity (fludarabine-busulfan) | Nonmyeloablative (fludarabine-cyclophosphamide-rituximab) | Nonmyeloablative (fludarabine-cyclophosphamide-rituximab) |
| Alternative donors a (%) | 59 | 37 | 63 | 50 | 0 |
| Relapse incidence (%) | 40 (4 y) | 38 (5 y) | 40 (5 y) | nr | 22 (3 y) |
| Progression-free survival (%) | 42 (4 y) | 39 (5 y) | 43 (5 y) | 36 (5 y) | 46 (3 y) |
| Overall survival (%) | 70 (4 y) | 50 (5 y) | 63 (5 y) | 51 (5 y) | 55 (3 y) |
| Follow-up (y) | 3.8 (0.6–8.5) | 5 (0.9–7.3) | 5.1 | 3.1 (0.9–10.9) | 2.3 (0.3–5.9) |
The most convincing proof of the GVL principle in CLL comes from studies analyzing the kinetics of minimal residual disease (MRD) after RIC alloSCT. MRD denotes a disease burden remaining after specific therapy, which is detectable only at a subclinical level. For CLL, this is defined as a contamination of 5 CLL cells or less per nanoliter of peripheral blood in the absence of clinical signs or symptoms of the disease. Patients showing less than 1 CLL cell in 10,000 benign leukocytes in peripheral blood or bone marrow are considered as being MRD negative.
In CLL, MRD can be quantified even at low levels by sensitive (>10E4) polymerase chain reaction– or flow cytometry–based assays. We analyzed MRD kinetics in 43 patients who had undergone RIC alloSCT within the prospective CLL3X trial of the German CLL Study Group (GCLLSG) or a pilot study and remained event-free 1 year after alloSCT. Of these, 32 patients had become MRD negative at 1 year after transplant, while in 11 patients the MRD level did not decrease below the threshold of detection until this landmark. Achievement of MRD negativity was clearly linked to immune intervention, such as tapering of immunosuppression or donor lymphocyte infusions ( Fig. 2 ). Absence of detectable MRD 1 year after alloSCT was strongly associated with a reduced risk of clinical relapse (hazard ratio [HR], 0.037; 95% confidence interval [CI], 0.008–0.18; P <.0001). Similar observations were reported by other investigators.
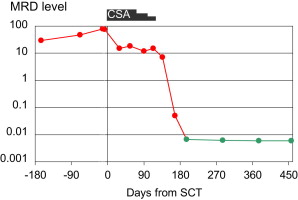
Altogether, MRD kinetics studies consistently indicate that permanent MRD negativity after alloSCT for CLL can be reached in the context of immunomodulating intervention. Both the durability of MRD remission and its sensitivity to immunomodulation strongly suggest that GVL activity is effective in CLL. GVL activity in CLL seems to be closely correlated to chronic GVHD, implying that it essentially depends on allogeneic effects with broader specificity rather than on a CLL-specific reactivity of donor GVL effector cells.
In summary, clinical trials and MRD studies provide clear evidence that GVL is working in CLL and can provide long-term MRD-free survival in up to 50% of patients undergoing RIC alloSCT.
Risks and tolerability of new alloSCT in CLL
The core feature of modern alloSCT strategies is that their tolerability has dramatically increased in comparison with traditional conditioning based on standard-dose total body irradiation or myeloablative doses of oral busulfan. Together with a tremendous improvement of supportive treatment (eg, antiemetics, antibiotics, and cytomegalovirus monitoring) during the past decade, this makes a huge difference for the patients especially during the early transplant phase (ie, conditioning, transplant, and aplasia): higher-grade nausea and mucositis affects only a small minority of patients undergoing RIC, and although grade 3 to 4 infections still occur in up to 60% of the patients, only few result in life-threatening complications ( Table 2 ). Accordingly, the early death rate (ie, death within the first 100 days after alloSCT) has dramatically decreased from up to 40% in the old days of traditional conditioning down to less than 3% with RIC (see Table 2 ). This rate has to be taken into account when considering the risk of dying with and without transplant. Thus, undergoing conditioning for alloSCT for CLL is no longer a “hell ride” with a 30% risk of not leaving the ward alive. It is clear that the prospective studies on RIC alloSCT represent selected patients and not intent-to-treat analyses from the time of relapse or transplant indication, implying that they should be biased in favor of a more favorable patient population in comparison to studies on nontransplant salvage trials in poor-risk CLL. However, even with this in mind it might be justified to state that with modern conditioning and appropriate supportive care, the hospitalization phase for alloSCT could be not less safe than other aggressive salvage therapies in patients with poor-risk CLL.
| Dreger et al, 2010 | Sorror et al, 2008 | Brown et al, 2012 | Khouri et al, 2011 | Michallet et al, 2011 | |
|---|---|---|---|---|---|
| N | 90 | 82 | 76 | 86 | 40 |
| Conditioning regimen | Nonmyeloablative (fludarabine-cyclophosphamide ± antithymocyte globulin) | Nonmyeloablative (fludarabine—low-dose total body irradiation) | Reduced intensity (fludarabine-busulfan) | Nonmyeloablative (fludarabine-cyclophosphamide-rituximab) | Nonmyeloablative (fludarabine-cyclophosphamide-rituximab) |
| Mucositis grade 3–4 (%) | 6 | 12 | nr | nr | <5 |
| Infection grade 3–4 (%) | 55 | 60 | nr | nr | 48 |
| Early death (within 100 d from alloSCT) (%) | 2 | <10 | <3 | 3 | 0 |
| Nonrelapse mortality (%) | 23 (4 y) | 23 (5 y) | 16 (5 y) | 17 (1 y) | 27 (3 y) |
| Acute GVHD 2–4 (3–4) (%) | 45 (14) | nr (16–23) | 30 (17) | 37 (7) | 44 (23) |
| Extensive chronic GVHD (%) | 55 | 49–53 | 48 | 56 | 29 |
| Follow-up (y) | 3.8 (0.6–8.5) | 5 (0.9–7.3) | 5.1 | 3.1 (0.9–10.9) | 2.3 (0.3–5.9) |
Despite the remarkable improvements in terms of early fatalities, nonrelapse mortality (NRM) after RIC alloSCT for CLL still mounts up to 15% to 25% during the first 2 posttransplant years. This scenario seems to be largely due to complications of acute and chronic GVHD. Although some studies suggest that patients exposed to RIC might be less prone to acute GVHD than those who underwent traditional myeloablative conditioning, higher-grade acute GVHD still affects up to 20% of patients after RIC alloSCT for CLL (see Table 2 ). Moreover, about half the surviving patients suffer from extensive chronic GVHD, which can contribute to late NRM.
Apart from its impact on NRM, chronic GVHD is the major determinant of long-term morbidity affecting quality of life (QOL) after alloSCT. At least 25% of survivors experience impaired life satisfaction during the first posttransplant years, with chronic GVHD being a robust predictor of reduced QOL. However, in many affected patients, clinical symptoms of chronic GVHD decrease over time. In their series of 82 patients, Sorror and colleagues observed a 5-year cumulative incidence of extensive chronic GVHD of 49% for related and 53% for unrelated recipients. Overall, in an increasing proportion of patients chronic GVHD resolved during follow-up, allowing discontinuation of therapeutic immunosuppression after a median of 25 months. Similar observations were made in the GCLLSG trial, in which one-third of the patients originally experiencing chronic GVHD were off systemic immunosuppression 1 year after transplant. Planned QOL analyses at baseline and 12 months postalloSCT were available for 24 of the patients who were event-free at 1 year after transplant. In spite of the high chronic GVHD risk, 58% of the patients reported the maximum QOL (Spitzer) score 12 months posttransplant (vs 48% at baseline), 32% of the patients reported an increase of their QOL score at 12 months compared with baseline, and only 14% reported a decreased score (GCLLSG, data on file).
In conclusion, transplant-related long-term morbidity after alloSCT for CLL can be significant but is mainly restricted to those patients who have ongoing active chronic GVHD. The morbidity caused by alloSCT for patients with poor-risk CLL must be weighed against the morbidity due to uncontrolled disease and palliative treatment associated with nontransplant salvage strategies.
It has to be emphasized that the good tolerability of RIC alloSCT allows offering the procedure to older (up to 70 years of age) and comorbid patients who represent the CLL target population. From 1991 to 2010, in the EBMT registry the proportion of patients who underwent RIC increased from 0 to more than 60% of all patients registered for alloSCT for lymphoma. During the same time interval, the fraction of patients older than 50 years who underwent allograft increased from 5% to 38%. Most recently, the 1-year NRM of the age group 61 to 70 years was still less than 25% ( Fig. 3 ).
In summary, RIC has resulted in a dramatic reduction of early morbidity and mortality of alloSCT in CLL, making this procedure now suitable for comorbid and elderly patients. Because up to 20% of patients who undergo allograft experience severe acute GVHD, long-term NRM in all prospective trials on RIC alloSCT still ranges from 15% to 25%. Long-term morbidity and QOL is largely determined by chronic GVHD (which is also the most important prerequisite for CLL eradication).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




