1. Serum LDH > 1.5 times the upper limit of normal
2. Hemoglobin level < lowest limit of normal
3. Corrected serum calcium level > 10 mg/dL (2.5 mmol/L)
4. Interval of less than 1 year from initial diagnosis of RCC to start of systemic therapy
5. Karnofsky performance score ≤ 70
6. ≥2 sites of organ metastases
3.3.2 Everolimus
Prior to reports of antitumor activity, everolimus was studied extensively in the setting of cardiac and renal transplantation. Antitumor effects were initially demonstrated in a rat pancreatic tumor model [59]. A single dose of everolimus was shown to block phosphorylation of 4E-BP1 and inactivate S6K1 in human peripheral blood mononuclear cells [59]. Everolimus is orally bioavailable with no active metabolites. A phase I dose escalation study demonstrated that everolimus was well tolerated at doses up to 70 mg weekly and 10 mg daily [60]. DLTs included hyperglycemia, stomatitis, and fatigue [60]. Partial responses were observed in 4 patients, and 12 patients remained progression-free for ≥6 months, including 5 of 10 patients with RCC [60]. Other phase I pharmacokinetic/pharmacodynamics studies showed that continuous daily dosing with everolimus 10 mg resulted in a more sustained targeted inhibition of mTOR than that achieved with a weekly dosage schedule [61, 62]. As a result, a daily dose of 10 mg was selected for further trials with everolimus.
A phase II study involving patients with mRCC, who had received at most one prior therapy other than an mTOR inhibitor, demonstrated the antitumor activity of everolimus 10 mg daily with reported median PFS and OS of 11.2 months and 22.1 months, respectively [63]. The pivotal phase III RECORD-1 trial examined the role of everolimus in patients with clear cell mRCC who had received prior sorafenib and/or sunitinib. This international study demonstrated that everolimus 10 mg daily resulted in a median PFS of 4.9 months compared to 1.9 months with placebo [26, 64]. Pharmacodynamic modeling of tumor growth in the RECORD-1 patient population showed that compared to placebo, everolimus 5 and 10 mg daily significantly slowed growth of mRCC target lesions, nontarget lesions, and new metastases; the 10 mg daily dosing was more effective than 5 mg daily in reducing growth of target lesions [65]. Based on results from the RECORD-1 study, oral everolimus was approved in the USA for patients with mRCC who had failed treatment with sunitinib or sorafenib and in Europe for patients who progressed on or after treatment with VEGF-targeted therapy [20, 22–24]. Although everolimus is well established as a second-line agent, its role as a first-line option is currently under investigation. The RECORD-3 trial is a phase II study investigating first-line everolimus followed by sunitinib versus standard sequence. Preliminary data demonstrated that PFS non-inferiority was not achieved with first-line everolimus when compared with sunitinib, supporting the current standard treatment paradigm [66].
3.4 Safety Considerations with mTORC-1 Inhibitors in Renal Cell Carcinoma
mTORC1 inhibitors are commonly associated with disorders of metabolism, noninfectious pneumonitis and stomatitis. Hyperglycemia and hypercholesterolemia are common although the severity is generally mild. Noninfectious pneumonitis has been recognized as a class effect of mTORC1 inhibitors. A follow-up study of patients treated with temsirolimus in the ARCC trial identified four cases of pneumonitis with one patient progressing from grade 3 to 5 toxicity [67]. The RECORD-1 trial reported that 14 % of patients treated with everolimus developed noninfectious pneumonitis [64]. Among ten patients who developed grade 3 noninfectious pneumonitis, eight had clinical resolution with steroid therapy. A review of these cases suggests that noninfectious pneumonitis can be managed effectively with early recognition and prompt intervention [68]. The use of imaging studies to monitor patients can be particularly challenging since radiographic abnormalities are seen in a higher percentage of patients receiving mTORC1 inhibitors compared to placebo in the absence of symptoms or a clinical diagnosis of pneumonitis [63, 67, 69]. Patients receiving mTORC1 inhibitors should be monitored closely for signs and symptoms of respiratory illness. Mild stomatitis and rash occurred in more than 20 % of patients in both the ARCC and RECORD-1 trials [25, 64]. These toxicities are manageable with standard supportive measures.
3.5 Limitations of mTORC1-Targeted Therapy
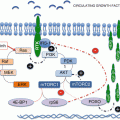
Although mTORC1 inhibitors produce clinically meaningful responses with improved PFS and OS, these responses are short-lived, and rarely do these therapies induce complete responses. None of the current available mTORC1 inhibitors have been able to induce sustained disease remission. Many patients initially respond but eventually relapse usually due to the development of resistance after a median of 6–15 months of treatment. These acquired mechanisms of resistance to mTORC1 inhibitors lead to reestablishment of tumor vasculature [70, 71]. They are thought to be facilitated through activation of alternative or compensatory pathways that lead to upregulation of various factors that promote cell growth and survival, including HIF. Potential mechanisms include transient and partial inhibition of 4E-BP1 and loss of negative feedback loops that are normally induced when mTORC1/p70S6K is active. The phosphorylation of 4E-BP1 has been shown to be less responsive to rapalogs than that of p70S6K. Although rapamycin inhibits the functions of p70S6K and 4E-BP1 in the short term, prolonged treatment renders mTORC1 to be rapamycin-resistant toward 4E-BP1 resulting in reinitiation of cap-dependent translation of mRNAs despite continued mTORC1 inhibition [72]. Findings by Choo et al. also suggest that catalytic inhibitors of mTOR, including a dual PI3K and mTOR inhibitor, were more effective than rapamycin in dephosphorylating 4E-BP1, supporting their clinical promise [72].
Recent data suggest loss of negative feedback loops from inhibition of mTORC1 leads to compensatory activation of PI3K and Akt which drives resistance via upregulation of mTORC2 [73]. Activation of S6K through mTORC1 phosphorylation results in phosphorylation of rictor, which prevents mTORC2 activation [74, 75]. If mTORC1/S6K is inhibited, the negative feedback is lost leading to derepression of mTORC2 and mTORC2-mediated phosphorylation and activation of Akt [76]. Activation of mTORC2 also leads to upregulation of HIF-2α which has been argued to be the more relevant HIF with respect to the development and progression of RCC. HIF-2α activation has been shown to strongly suppress E-cadherin expression, allowing for increased cell motility [77]. E-cadherin loss is frequently associated with tumor progression and metastasis [78]. These findings highlight the potential therapeutic advantage of simultaneous inhibition of mTORC1 and mTORC2 in preventing tumor cell proliferation, growth, invasion, and metastasis.
Another potential mechanism of resistance is the loss of a negative feedback loop that normally prevents upstream overstimulation of insulin receptor substrate 1 (IRS1)/PI3K/Akt signaling [79]. mTORC1 activation of S6K causes destabilization of IRS1-2 which uncouples IGF-1 from the PI3K/Akt pathway. Normally, IGF-1 binds IGFR which in turn phosphorylates substrates IRS1-2 which then relays the activation to PI3K. mTORC1/S6K inhibition results in the loss of this feedback loop and leads to the upregulation of IRS1 protein and activation of the PI3K/Akt cascade [80]. PI3K/Akt signaling activates an array of kinases that promote cell growth and survival. This prosurvival effect occurs through various pathways including negative regulation of factors that promote expression of death genes, positive regulation of prosurvival genes such as NF-κB, direct phosphorylation and inactivation of proapoptotic proteins, and regulation of the cell cycle [81].
3.6 Future Directions and Novel Therapies
Because of their suspected roles in resistance to mTORC1 inhibitors, PI3K, Akt, and mTORC2 are potential targets for the development of novel therapies for various malignancies, including mRCC. Consistent with their proposed roles in the development of resistance and pathogenesis of mRCC, a microarray analysis of RCC tissue specimens showed that high PI3K and mTOR expression levels corresponded with late-stage, high-grade tumors and were prognostic factors for decreased survival [82]. A number of PI3K, mTORC1/2, and Akt inhibitors have been developed and have demonstrated promising results in RCC cell lines and xenograft models. This section will focus on these novel targeted agents that have been evaluated in RCC (Table 3.2).
Table 3.2
Novel agents targeting PI3K/Akt and mTOR pathways in development
Agent | Target | Formulation | Phase of development | Drug company | Reference |
|---|---|---|---|---|---|
INK128/MLN0128 | mTORC1/2 | Oral | Phase I | Intellikine | |
WYE-125132 | mTORC1/2 | Oral | Preclinical | Wyeth | [87] |
AZD8055 | mTORC1/2 | Oral | Phase I | AstraZeneca | |
Ku0063794 | mTORC1/2 | Intravenous | Preclinical | Kudos Pharmaceuticals | [92] |
NVP-BEZ235 | PI3K/mTORC1/2 | Oral | Phase I/II | Novartis | |
SF1126 | PI3K/mTORC1/2 | Oral | Phase I | Semaphore Pharmaceuticals | |
BKM120 | PI3K | Oral | Phase I/II | Novartis | |
Perifosine | Akt | Oral | Phase II | Keryx Biopharmaceuticals | |
MK2206 | Akt | Oral | Phase II | Merck |
3.6.1 mTORC1/2 Inhibitors
Novel mTORC1/2 inhibitors bind directly to the adenosine triphosphate (ATP)-binding domain of mTOR, resulting in the inhibition of both mTORC1 and mTORC2 (Table 3.2) [83, 87, 88, 92]. These mTOR kinase inhibitors prevent the rebound activation of PI3K/Akt cascade as seen with rapalogs. An mTORC1/2 inhibitor can also prevent HIF-2α suppression of E-cadherin expression and result in restored cell-cell adhesion to prevent tumor cell motility and migration [77]. INK128/MLN0128 is a highly potent, orally active mTOR kinase ATP-competitive inhibitor that is currently being investigated in RCC cell lines [83]. Preclinical data suggest that it has antitumor and antimetastatic activity in prostate cancer models as well as synergistic activity with TKI lapatinib in breast cancer models refractory to anti-HER2 therapy [84, 85]. INK128/MLN0128 has been shown to inhibit downstream substrates of mTOR, phosphorylation of Akt, and tumor cell proliferation as well as induce G1 cell cycle arrest [86]. INK128/MLN0128 demonstrated antitumor activity in RCC mouse models which was further enhanced in combination with sorafenib or bevacizumab. The combination resulted in a sustained regression of the tumor through inhibition of tumor cell proliferation by INK128/MLN0128 and angiogenesis by sorafenib/bevacizumab [83]. These findings suggest that combination therapy may be an option for maximizing therapeutic benefits of novel agents in the treatment of mRCC.
WYE-125132 is a pyrazolopyrimidine molecule that acts as an orally active, highly potent, ATP-competitive and specific mTOR kinase inhibitor. It has demonstrated antitumor activity in RCC cell lines and mouse models resulting in strong G1 phase arrest and tumor growth suppression [87]. Combination of WYE-125132 and bevacizumab caused dramatic tumor regression of large A498 tumors [87]. Unlike rapalogs, WYE-125132 was able to disrupt cap-dependent translation initiation eIF4F complex; after treatment with the molecule, there was a drastic increase in the inhibitory binding of 4E-BP1 to eIF4E with almost complete loss of eIF4G. WYE-125132 also strongly inhibited hypoxia-induced accumulation of HIF-1α and HIF-2α [87].
AZD8055 is a third potent, orally active, highly selective mTORC1/2 inhibitor. Preclinical data show that it is better at inhibiting phosphorylation of 4E-BP1 than rapamycin, resulting in significant inhibition of cap-dependent translation [88, 89]. It was also able to inhibit Akt in MCF-7 breast carcinoma cells where rapamycin treatment resulted in rebound activation of Akt [88, 89]. Chresta et al. demonstrated that AZD8055 potently inhibits cellular proliferation and induces autophagy in vitro with H838 and A549 cells. In vivo, AZD8055 induced significant tumor growth inhibition and regression in a variety of human tumor types, including breast, lung, colon, prostate, and uterine xenograft models [89]. Recent data suggest that AZD8055 has significant antitumor activity against clear cell RCC cell lines UOK-139 and UOK-140 [90]. AZD8055 is currently undergoing clinical evaluation in phase I trials. Naing et al. reported a maximum tolerated dose of 90 mg PO BID. DLTs included grade 3 transaminitis (increased alanine aminotransferase 22 %, increased aspartate aminotransferase 22 %) and fatigue (16 %) [91]. Transaminitis was reversible in all patients, except for one with liver metastases. AZD8055 was overall well tolerated, but no complete or partial responses were observed [91].
Ku0063794 is another highly specific small molecular inhibitor of mTOR kinase. It has been shown to inhibit phosphorylation of S6K and 4E-BP1 as well as Akt phosphorylation [92]. Ku0063794 has been compared with temsirolimus in preclinical RCC models. It was found to be more effective than temsirolimus in decreasing viability and growth of RCC cell lines in vitro by inducing cell cycle arrest and autophagy, but not apoptosis [92]. However, in xenograft models, there was no difference in the inhibition of tumor growth by Ku0063794 or temsirolimus [92]. A potential explanation is that temsirolimus has additional effects on tumor microenvironment, including decreasing tumor angiogenesis. VEGF and PDGF expression was lower in cells treated with temsirolimus than in cells treated with Ku0063794 [92]. This observation suggests that mTORC1/2 inhibitors may provide better tumor suppression and regression in combination with anti-angiogenic agents.
3.6.2 PI3K/mTOR Inhibitors
Because the catalytic domain of mTOR and p110α subunit of PI3K is structurally similar, multiple agents have been developed to have dual inhibitory activity against PI3K and mTORC1/2 (Table 3.2) [82, 93]. These ATP-competitive, pan-selective inhibitors of PI3K and mTOR have demonstrated impressive antitumor activity in a wide range of tumor models. NVP-BEZ235 is a potent orally available imidazoquinoline dual PI3K/mTOR inhibitor. It reversibly inhibits class 1 PI3K activity by binding to its ATP-binding domain [94]. It also directly binds to the mTOR ATP-binding domain and inhibits its catalytic activity. In preclinical studies, NVP-BEZ235 has been shown to inhibit PI3K and mTOR activity resulting in tumor growth suppression in numerous human tumor models, including glioblastoma, multiple myeloma, and prostate, breast, and pancreatic carcinoma [95–97]. A comparison of NVP-BEZ235 and rapamycin activity in RCC xenografts revealed that NVP-BEZ235 is significantly more effective at downmodulating cyclin D, survivin, and HIF-2α than rapamycin. It was also more effective at inhibiting tumor growth both in vitro and in vivo through antiproliferative and proapoptotic effects [93]. A study with RCC cell lines 786-O and Caki-1 demonstrated that the combination of NVP-BEZ235 and sorafenib had greater antitumor activity through reduction of tumor cell growth and increasing apoptosis than either agents alone [98]. This finding suggests that dual PI3K/mTOR inhibitor in combination with an anti-angiogenic agent may result in enhanced synergistic antitumor activity. A phase I clinical trial with advanced solid tumors showed that BEZ235 is generally well tolerated with a favorable safety profile [99]. The most commonly reported adverse events included nausea, vomiting, diarrhea, fatigue/asthenia, and anorexia. Available pharmacodynamics and efficacy data also showed that NVP-BEZ235 is active, especially in patients with PI3K pathway dysregulated tumors [99]. Another phase I study with a new formulation of NVP-BEZ235 using a solid dispersion system (SDS) sachet included three RCC patients and showed that this specific formulation was well tolerated [100]. Common adverse events included nausea, vomiting, diarrhea, and fatigue/asthenia. The SDS sachet formulation of NVP-BEZ235 has been chosen for further evaluation in phase II clinical trials [100].
Another pan-PI3K/mTORC inhibitor SF1126 is a prodrug of LY294002 administered intravenously. The active LY294002 has significant antitumor and anti-angiogenic activities in vivo, but is not a drug candidate due to insolubility and short half-life. To increase solubility and bioavailability, LY294002 is conjugated to RGD (Arg-Gly-Asp) peptide via a cleavable linker to form SF1126. In preclinical models, SF1126 exhibited both antitumor and anti-angiogenic activities [101]. In a 786-O RCC xenograft model, SF1126 demonstrated 50–90 % tumor inhibition or regression of tumor volume [101]. It has also been shown to significantly suppress signaling pathways downstream of PI3K, including Akt, and eliminate hypoxia-induced stabilization of HIF-2α [102]. A phase I clinical trial found that SF1126 is generally well tolerated [103]. Grade 3 DLTs included peripheral edema, increased alkaline phosphatase, diarrhea, weakness, hypoglycemia, urticaria/pruritus, anemia, hypokalemia, and hypersensitivity [103]. Common adverse events included nausea, fatigue, vomiting, diarrhea, pyrexia, chills, pruritus, anemia, anorexia, and headache [103]. Stable disease was the best response observed with mean duration of 21 weeks (range of 8–84 weeks); 2 of the 3 RCC patients had stable disease at 14 and 84 weeks [103].
3.6.3 PI3K Inhibitors
In addition to mTORC1/2 inhibitors and dual PI3K/mTOR kinase inhibitors, PI3K-selective inhibitors are currently under investigation (Table 3.2). BKM120 is an oral pyrimidine-derived pan-PI3K inhibitor with specific and potent activity against class I PI3Ks [104, 105]. In preclinical studies, BKM120 demonstrated a strong antiproliferative effect and induced apoptosis in vitro on various human cancer cell lines [105]. In vivo, BKM120 had significant antitumor activity in U87MG glioblastoma and A2780 ovarian xenograft models [105, 106]. A phase I study showed that BKM120 is well tolerated with median treatment duration of 7.5 weeks and showed antitumor activity in 28 of 66 patients, including 2 patients with partial response and 26 with stable disease [104]. Adverse events included decreased appetite, rash, diarrhea, nausea, fatigue, hyperglycemia, anxiety, depression, and mucositis [104]. BKM120 is currently being tested in a number of clinical trials, including a phase I study in combination with bevacizumab in patients with mRCC who had failed prior systemic therapies.
3.6.4 Akt Inhibitors
Because of Akt’s critical role in cellular survival and tumorigenesis, Akt inhibitors have been developed with promising results (Table 3.2). Perifosine is a synthetic, substituted heterocyclic alkylphospholipid with the ability to inhibit Akt activity [107]. It inhibits Akt activation by interfering with the interaction between the pleckstrin homology domain of Akt and phosphatidylinositol phosphate (PIP3) [107]. This interference precludes Akt’s translocation to the plasma membrane where activation would have occurred through phosphorylation by pyruvate dehydrogenase kinase, isozyme 1 (PDK1). Fu et al. showed that perifosine induced autophagy and inhibited assembly of the mTOR complexes by promoting degradation of Akt, mTOR, rictor, raptor, p70S6K, and 4E-BP1 [108]. A phase I trial showed that perifosine was well tolerated with nausea, vomiting, diarrhea, and fatigue as the most commonly observed toxicities [109]. A phase II trial assessed the efficacy and safety of perifosine in patients with advanced RCC who had failed previous VEGF-targeted therapy. It demonstrated modest activity in patients with advanced RCC, but this activity was not superior to currently available second-line agents [110]. Further studies are needed on the possibility of combination therapy with perifosine for RCC.
MK-2206 is a potent orally active allosteric Akt inhibitor. It has nanomolar potency against purified recombinant human Akt1 (half maximal inhibitory concentration [IC50], 5 nmol/L) and Akt2 enzymes (IC50, 12 nmol/L) but lower potency against human Akt3 (IC50, 65 nmol/L). MK-2206 inhibits phosphorylation at Thr308 and Ser473 of AKT and demonstrates greater than 100-fold selectivity of Akt against more than 200 other kinases [111]. It has in vitro and in vivo antitumor activity as a single agent and enhances preclinical activity of conventional cytotoxic chemotherapy and other targeted therapies [112, 113]. Hirai et al. demonstrated that MK-2206 synergistically inhibited cell proliferation in combination with molecular targeted agents, such as erlotinib and lapatinib as well as with standard cytotoxic agents, including doxorubicin, gemcitabine, 5-fluorouracil, docetaxel, and carboplatin in lung NCI-H460 and ovarian A2780 cells [113]. In vivo, the addition of MK-2206 exerted significantly more potent antitumor activity than each agent in the monotherapy setting [113]. A phase I clinical trial involving 33 patients with advanced solid tumors, including patients with RCC, showed that MK-2206 was well tolerated [114]. DLTs included skin rash and stomatitis. The maximum tolerated dose was established at 60 mg. Drug-related toxicities included skin rash (51.5 %), nausea (36.4 %), pruritus (24.2 %), hyperglycemia (21.2 %), and diarrhea (21.2 %) [114]. Another phase I study investigated the maximum tolerated dose, DLTs, PK, and efficacy of MK-2206 in combination with targeted and cytotoxic agents in patients with advanced solid tumors, including patients with RCC [115]. MK-2206 with carboplatin/paclitaxel, docetaxel, or erlotinib was found to be well tolerated. DLTs included skin rash, febrile neutropenia, tinnitus, and stomatitis. Common adverse events included fatigue (68 %), nausea (49 %), rash (47 %), diarrhea (44 %), anorexia (44 %), alopecia (40 %), vomiting (36 %), stomatitis (32 %), and hyperglycemia (25 %) [115]. A recent phase II clinical trial compared MK-2206 with everolimus in patients with VEGF inhibitor refractory mRCC [116]. MK-2206 was held in three patients due to grade 3 rash, and one patient had to come off study for the rash. Median PFS for MK-2206 was 3.65 months and 7.43 months for everolimus. Two patients in the MK-2206 group demonstrated dramatic responses with greater than 50 % disease regression and PFS of 8 and 6 months. Jonasch et al. showed that monotherapy with MK-2206 was not superior to everolimus, but a dramatic response to MK-2206 was seen in a subset of patients [116]. Further translational studies analyzing genotype-phenotype correlations may help explain this observation and identify biomarkers to allow for patient selection and rational drug combination.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree