• Prostate cancer cells usually grow more slowly than many other malignancies, thus allowing time for the elicitation of effective immune responses able to translate into clinical benefit
• Biochemically recurrent prostate cancer provides an unique opportunity for immunological intervention, as the multiple immunosuppressive mechanisms associated with an advanced tumor burden are expected to be at a minimum at this stage
• Prostate cells express many a number of specific proteins that could act as immune therapeutic targets, including prostate-specific antigen (PSA), prostatic acid phosphatase (PAP), and prostate-specific membrane antigen (PSMA)
Sipuleucel-T
As discussed above, adaptive immune responses are primarily mediated by dendritic cells (DC), and a cancer vaccine, like any other vaccine, would be expected to depend on the presence of a population of host DC that are numerically and functionally intact. Unfortunately, this is often not the case in cancer patients, in which DC are often dysfunctional [16]. One approach to overcoming this dysfunction, then, might be to generate new autologous DC outside of the patient’s tolerogenic environment. This is indeed the approach involved in the generation of Sipuleucel-T, which was the first cancer vaccine approved by the FDA for the treatment (rather than prevention) of cancer. The product is individually manufactured for each patient with PC, in a process which comprises multiple steps (Fig. 9.1a). Briefly, patients undergo a standard leukopheresis procedure and peripheral blood mononuclear cells (PBMC) are separated and then incubated with PAP2024, a fusion protein that links the antigen PAP to the granulocyte-macrophage colony-stimulating factor (GM-CSF). After approximately 36 h of incubation, cells are washed and suspended in Lactate Ringer Injection, for infusion back into the patient. In this approach, the GM-CSF serves as the adjuvant that helps to activate dendritic cells and other cells in the infusion product. The process is repeated three times at 2-week intervals [17].
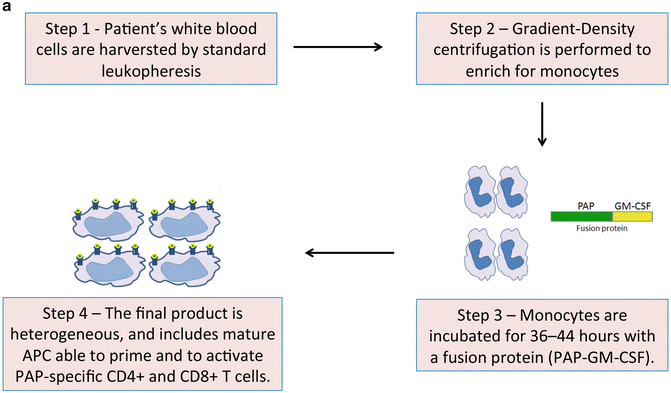
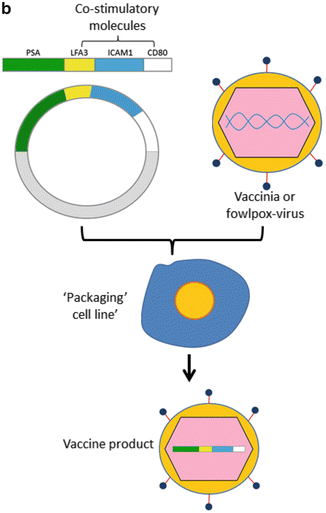
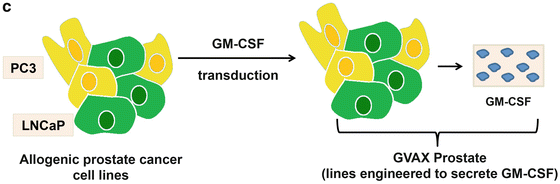



Fig. 9.1
(a) Sipuleucel-T: The figure outlines the steps involved in manufacturing Sipuleucel-T, an autologous vaccine product which is administered intradermally. (b) ProstVac VF: As detailed in the text, this is a viral-based vaccine directed against PSA. The construct includes three costimulatory molecules designed to augment an adaptive immune response. ProstVac VF is administered subcutaneously and is an off the shelf product with is not patient specific. (c) GVAX prostate: GVAX prostate consists of two allogeneic prostate cancer cell lines transduced to secrete GM-CSF. The vaccine product is irradiated and injected intradermally, where cells undergo necrosis and are taken up by antigen-presenting cells resident in the skin. Vaccine antigens (multiple) are taken up and presented to T cells to initiate an adaptive immune response, theoretically poly-antigenic in nature
Clinical Data (Sipuleucel-T)
After a series of phase I and II trials, two relatively small randomized, double-blind, placebo-controlled phase III trials (D9901 and D9902A) showed evidence for the clinical activity of Sipuleucel-T [18]. Both studies enrolled men with asymptomatic or minimally symptomatic mCRPC, and both suggested clinical activity via an increased survival. Thus, the multicenter, randomized, double-blind, placebo-controlled IMPACT trial was designed with primary endpoint of OS. trial trail enrolled 512 patients with asymptomatic or minimally symptomatic mCRPC randomized 2:1 to receive Sipuleucel-T or placebo. Resulting data showed that treatment with Sipuleucel-T was increased median OS by 4.1 months (25.8 versus 21.7 months; p = 0.032, HR = 0.78) [19]. As in the prior phase III studies, median time to progression was not different between groups, and radiographic responses were not generally observed. Sipuleucel-T was generally well tolerated, and 92 % of patients received all three infusions. The most common adverse events (AEs) in the Sipuleucel-T group included chills (in 51.2 %), fever (22.5 %), fatigue (16.0 %), nausea (14.2 %), and headache (10.7 %); all these were graded as mild or moderate. Adverse events of grade 3 or more within 1 day after infusion were rare, and were reported in 23 of 338 patients (6.8 %) in the Sipuleucel-T group and 3 of 168 patients (1.8 %) in the placebo group.
Mechanism of Action
The final Sipuleucel-T product is heterogeneous, comprising mature antigen-presenting cells (APC) and other cell types, including T cells, B cells, and natural killer cells [17]. Once infused, the autologous ex vivo-activated APC are thought to prime PAP-specific CD4+ and CD8+ T cells in a manner similar to a classical vaccine-mediated prime-boost regimen, where the first infusion primes the immune system and subsequent infusions boost the response [20]. Recently, a combined analysis of immunological data from the previously cited phase III trials (D9901, D9902A, and IMPACT) was completed [21]. The authors demonstrated that APC activation in the infused product occurred with the initial dose, and increased with subsequent doses. The median cumulative APC activation with Sipuleucel-T across the three dose preparations was 26.7 [95 % CI 21.5–33.6]. Furthermore, these data demonstrated antigen-specific T-cell proliferation and IFN-γ ELISPOT activity in pre-culture cells obtained at weeks 2 and 4 (but not week 0). Finally, T-cell activation-associated cytokines were noted in the second and third doses of Sipuleucel-T, supporting the idea that the first infusion of activated, antigen-loaded APCs primes T cells in vivo. Thus, the second and third doses are of Sipuleucel-T are biologically different from the first, and each dose contains progressively more activated APCs and possibly a greater proportion of antigen-specific T-cells with the capacity to recognize and kill PC cells. Based on these studies, antigen-specific immune responses, in the form of either PAP-specific antibodies, or a T cell response to PAP were observed in 78.8 % of monitored subjects. Finally, the authors demonstrated a positive correlation between OS and cumulative APC activation and antigen-specific immune responses. Taken together these important analyses show that Sipuleucel-T induces an antigen-specific immune response, and that response appears to be associated with a survival benefit in treated patients.
ProstVac-VF
ProstVac VF is a poxvirus-based vaccine directed against PSA (Bavarian Nordic, USA); this technology was developed over several years by a group at the NIH [22], and its current iteration includes a number of critical modifications to optimize immunogenicity. First, the vaccine involves a heterologous prime boost in which the initial vaccine is based on a modified vaccinia ankara (MVA) backbone, followed by a series of booster vaccines with a fowlpox backbone. This is because the immune response to the MVA backbone is quite robust, so boosting with an identical vaccine is limited by the host’s immune response to the viral backbone. To further increase immunogenicity, the vaccine was engineered to incorporate a triad of costimulatory molecules, including leukocyte function-associated antigen 3 (LFA-3), the T cell costimulatory molecule B7.1, and the adhesion molecule intercellular adhesion molecule 1 (ICAM-1) [23] (Fig. 9.1b). Additionally, administration of GM-CSF at the vaccination site is used to help recruit local dendritic cells (APC) and enhance antigen presentation.
Clinical Data (ProstVac-VF)
Aside from the multiple combination trials performed using ProstVac VF (see below), two randomized phase II single-agent trials in men with mCRPC provided important evidence for clinical activity. In one of these trials, 32 patients with mCRPC were enrolled, and antigen-specific immune PSA responses were shown to be associated with survival [24]. Additionally, 12 of 32 patients showed declines in serum PSA post-vaccination and 2/12 showed radiographic regression of index lesions. A second multicenter, randomized, double-blind study enrolled 125 patients randomized 2:1 to receive PROSTVAC-VF plus GM-CSF or control vectors [25]. This trial was designed with primary endpoint of time to disease progression. Although the study did not meet its primary endpoint, a significant survival advantage was observed; at 3 years post study, ProstVac-VF patients had a better OS with 25 (30 %) of 82 alive versus 7 (17 %) of 40 controls, a longer median survival (25.1 versus 16.6 months for controls, HR = 0.56, p = 0.0061). As in prior trials, the therapy was well tolerated and the most common AEs were injection site reactions. Systemic AEs, such as fatigue, fevers, and nausea, were reported in a subset of patients. Based on the survival benefit showed in these trials, an international randomized phase III trial has recently been initiated. This trial (NCT01322490, PROSPECT) will enroll 1,200 patients and will randomize them to either placebo, ProstVac-VF plus subcutaneous GM-CSF, or to ProstVac-VF alone. The primary endpoint of this trial is overall survival, and enrollment is limited to men with asymptomatic or minimally symptomatic mCRPC who are chemotherapy naive.
Mechanism of Action (ProstVac VF)
In vivo, poxvirus vectors most likely infect epithelial cells, a proportion of which undergo cell death. Cellular debris, including encoded antigens, are then taken up by nearby immature APCs, which, when appropriately activated, can present these antigens to CD4+ and CD8+ T cells in a pro-inflammatory context [20]. This process is known as indirect or cross-priming. Direct infection of APCs, particularly the Langerhans cells in the skin, is another mechanism by which poxvirus vectors can prime an immune response [20], and the relative role of direct versus cross-priming in patients treated with ProstVac-VF is currently not known. Nevertheless, the end result is postulated to be activation and proliferation of PSA-specific CD8 and CD4 T cells, as has been demonstrated in earlier correlative studies. Interestingly, and in contrast to the recently published data on Sipuleucel-T [21], ProstVac-VF doesn’t appear to prime much of an antibody response; indeed antibodies specific for PSA have not been reported with this agent.
GVAX Prostate
GVAX prostate (Aduro Biotech, Berkeley, CA) is a cell-based immunotherapy comprised of a combination of irradiated allogeneic tumor cell lines modified with granulocyte-macrophage colony-stimulating factor (GM-CSF) gene [26] (Fig. 9.1c). The product includes two prostate carcinoma cell lines: the androgen-sensitive LNCaP, as well as the castration-resistant line PC3 [27]. The concept underlying whole-cell vaccines is that these cells will serve as a diverse source of multiple tumor and tissue specific antigens, at least some of which will correspond to antigens in the patient’s tumor. These vaccines are thus considered polyvalent. The primary advantage to such an approach is that it’s possible that the inclusion of multiple antigens might prevent tumors from escaping immune pressure by down-regulating the expression of a single tumor-associated antigen. The major disadvantage of GVAX vaccines is that they’re relatively difficult to monitor immunologically, since the key target antigen/antigens are not known for any particular patient.
Clinical Data (GVAX Prostate)
Similar to both Sipuleucel-T and ProstVac-VF, two relatively small phase I/II trials of GVAX prostate verified safety and showed some early evidence for clinical activity. The first of these trials [28] enrolled 55 patients with chemotherapy naïve mCRPC. In men with radiographically documented metastases, the median survival in a group of men receiving a high dose of vaccine (N = 10) was 34.9 months, versus 24 months in 24 men receiving a lower dose of the vaccine. No dose limiting toxicities were noted, although injection site reactions, often prominent, were commonly observed. In a second dose-escalation trial [29], 80 men were treated, and PSA stabilization was observed in 19 % of patients, with a single patient showing a 50 % decline in PSA. Based on these trials, two multicenter randomized phase III trials were initiated. The first of these trials (VITAL-1) was a 626 patient trial in which men with asymptomatic mCRPC were randomized 1:1 to GVAX prostate (q 2 weeks × 13 doses) [30]. Interestingly, the comparator arm in this trial was not placebo, as in the ProstVac VF and Sipuleucel-T trials. Rather, standard dose docetaxel (75 mg/m2 × 10 doses + prednisone 5 mg BID) was chosen as the comparator arm. The primary endpoint of the trial was overall survival, but the trial was halted prematurely based on an unplanned and underpowered futility analysis. Thus, it remains unknown whether GVAX prostate is capable of providing a survival advantage in men with early stage mCRPC. A second trial, Vital-2 compared the combination of GVAX prostate + chemotherapy (without prednisone) versus docetaxel chemotherapy (with prednisone) was initiated, but enrollment was halted based on a perceived imbalance in deaths, with 67 on the GVAX + chemotherapy arm, versus 47 on the chemotherapy alone arm. The trial was later closed on the basis of that “imbalance,” but follow-up data showed that, in a final analysis, there was no statistical imbalance in deaths, with 85 on the combination arm and 76 in the chemotherapy alone arm. Taken together, the two GVAX trials both support the safety of these cell-based vaccines in prostate cancer, but unfortunately no conclusions can be drawn about efficacy since both trials were halted before meeting pre-specified enrollment criteria. An interesting facet of both trials is the choice of chemotherapy as a comparator arm; no other vaccine trial either before or after this has chosen chemotherapy as a comparator. At the current time, a single GVAX prostate trial is underway (NCT01696877), this is a neoadjuvant trial designed to test the ability of the vaccine to induce a T cell influx into [31] the prostate gland when administered along with hormonal therapy in the pre-surgical setting.
Mechanism of Action (GVAX Prostate)
GVAX prostate is thought to function in a manner similar to ProstVac VF—irradiated cells are administered intradermally, where they undergo necrosis and are taken up by resident dendritic cells attracted by the GM-CSF. After uptake of cellular debris and processing, antigens are presented to host CD4 and CD8 T cells in the context of host MHC molecules on the APC. This process, cross-presentation, has been demonstrated clinically [31]. As noted above, cell-based vaccines such as GVAX have the theoretical advantage of being able to present multiple tumor-related antigens simultaneously.
Prostate Cancer Vaccines in Clinical Practice
Although the focus of this chapter is the management of metastatic castration-resistant prostate cancer (mCRPC), and this is the setting where the majority of clinical trials of cancer immunotherapy have been performed, from an immunological perspective, it is important to highlight that biochemical recurrence provides a unique opportunity for immunological intervention in patients with prostate cancer. In this setting the several of the immunosuppressive mechanisms (such as Treg cells, myeloid-derived suppressor cells (MDSCs) and transforming growth factor-β (TGFβ)) associated with an advanced tumor burden are expected to be at a minimum, and it is likely that immunotherapy approaches would be more beneficial [20]. But it must be noted that the single prostate cancer vaccine that is FDA approved (Sipuleucel-T) is approved only in the metastatic state, and that clinical development of prostate cancer drugs is somewhat hampered by the absence of clear markers of response in the non-metastatic setting.
Among men with mCRPC, then, which patients are most appropriate for immunotherapy with Sipuleucel-T, and perhaps more importantly, when in the treatment sequence should this agent be given? In terms of patient status, the enrollment criteria for all 3 phase III trials of this agent stipulated that men be either asymptomatic or minimally symptomatic, so it’s clear that men with advanced, symptomatic disease are most likely not appropriate candidates. In terms of sequencing, it is not well appreciated that approximately 20 % of the men treated in the phase III trial of Sipuleucel-T (IMPACT) had been treated with prior chemotherapy [19]. Subgroup analysis suggested a benefit in both chemotherapy-experienced and chemotherapy-naïve patients, so prior chemotherapy in and of itself is not an absolute contraindication to vaccine treatment, as long as patients are either asymptomatic or minimally symptomatic. Perhaps more relevant at the current time are the second-generation hormonal treatments such as abiraterone-acetate [32] and enzaluatmide [33]. These are well-tolerated agents, often administered in the pre-chemotherapy setting. Their sequencing with immunotherapy has not yet been well studied, and in the case of abiraterone acetate a particular concern arises from the notion that this agent is usually administered with a low dose of prednisone (5 mg BID). Recent data from a randomized phase II trial of Sipuleucel-T administered either concurrently (N = 30) or sequentially (N = 32) with Abiraterone acetate + prednisone (AA + P) suggested that the administration of AA + P did not appear to influence the parameters of Sipuleucel-T associated with potency (CD54 count), and that evidence for a prime-boost effect was still documented in the product in the presence of prednisone [34]. While clinical follow-up of these patients continues, the data provide important evidence that co-administering AA + P is not obviously contraindicated. Still, though, it’s not clear whether the first therapy for men with progressing mCRPC should be Sipuleucel-T or a second-generation hormonal agent. Clearly, large randomized clinical trials, following men for overall survival would be necessary to answer that question definitively. Those trials are not currently underway, and are in fact unlikely to be initiated. A second set of data, albeit retrospective, add a bit of additional insight. In a retrospective analysis of the large randomized phase III trial of Sipuleucel-T, patients were subgrouped based on initial PSA, and those data correlated with potential survival benefit [35]. In this study, patients with the lowest quartile of initial PSA (<22.2 ng/mL) appeared to enjoy the largest survival benefit from Sipuleucel-T (41.3 versus 28.3 months for placebo), while patients with the highest quartile of initial PSA (>134.1) appeared to achieve a less obvious survival benefit (18.4 months with Sipuleucel-T versus 15.6 months for placebo). So, taken together these data would suggest that the most appropriate patients for Sipuleucel-T treatment would be those with early stage disease, a lower PSA, and fewer symptoms.
GM-CSF as a Treatment Modality
As discussed above, GM-CSF is a component of all three vaccine approaches that have been evaluated in prostate cancer. As a cytokine, GM-CSF the most relevant role of GM-CSF is the recruitment of APC to a vaccine site, but it also has a role in APC activation. It should be noted, however, that the role of GM-CSF is not completely clear, as some studies showed that higher concentrations can lead to the induction and proliferation of a population of cells with immune suppressive properties known as Myeloid-Derived Suppressor Cells (MDSC) [36]. Whether GM-CSF induces MDSC in human cancer patients is less clear, but several studies with peptide vaccines showed that the addition of GM-CSF does not seem to significantly augment vaccine efficacy, and may in fact impair it [37]. Possibly based on those data, the phase III trial of ProstVac VF includes two vaccine arms, one with and one without GM-CSF. As an FDA-approved agent with immunological properties, GM-CSF has been tested as a single-agent in men with prostate cancer. In the first of these trials [38], GM-CSF was administered at dose of 250 mcg/m2/day on days 1–14 of a 28-day cycle to 30 patients with biochemically recurrent prostate cancer. Approximately 10 % of the patients had a 50 % decline in PSA, and the rate of PSA rise (PSA slope) appeared to decrease in the group as a whole, suggesting an alteration in disease kinetics. Longer term follow-up of these patients showed that seven out of the initial 30 patients enrolled (24 %) remained free of disease progression a median of 5 years after starting therapy, and that those responding patients tended to have lower Gleason scores and pretreatment PSA values [39]. Despite these interesting and encouraging results, GM-CSF monotherapy in mCRPC has not been more extensively followed up in a large randomized trial setting, and the agent is not specifically FDA-approved for prostate cancer. It’s worth mentioning that GM-CSF treatment has also been combined with immune checkpoint blockade using anti-CTLA-4 (see below), and here PSA responses were seen at antibody doses of 3 mg/kg and above [40].
Immune Checkpoint Blockade
As discussed above, T cells have a crucial role in anti-tumor immunity. Recent developments in basic immunology show that T cell function is physiologically modulated by the interaction of a series of cell surface proteins (called immune checkpoints) with their individual ligands [3, 41, 42]. In normal physiology, these molecules likely serve to prevent an over-exuberant immune response to infection from causing autoimmunity, but in the case of cancer these molecules likely attenuate and/or prevent an anti-tumor immune response. Perhaps the best studied of the checkpoint molecules is Cytotoxic T Lymphocyte Antigen-4 (CTLA-4), originally cloned as an analog of the costimulatory molecule CD28. While the function of CTLA-4 was controversial for some time [43], definitive studies using knockout mice were performed by a number of groups, with concordant results. Mice with germline deletion of CTLA-4 develop widespread lymphadenopathy and autoimmunity, and die within 21–28 days of age, attesting to a critical role for this molecule in attenuating a T-cell driven immune response [44, 45]. Subsequent studies by the Allison group showed that blocking CTLA-4 with a monoclonal antibody could potentiate anti-tumor immunity in an animal model [46]. A monoclonal anti-CTLA-4 antibody was developed clinically, and is now FDA-approved for patients with metastatic melanoma [47].
Mechanism of Action (Anti-CTLA-4)
As show in Fig. 9.2, T cell activation is a carefully orchestrated process which requires at least two distinct signals. The first signal, Signal 1, is the cognate interaction between a T cell’s unique cell surface receptor (the TCR) and a surface formed by the combination of a MHC molecule and a particular peptide. The TCR signal is initiated with this is of sufficient affinity to qualify as a “good fit.” But signal-1 alone is insufficient for full T cell activation, indeed T cells require a second signal, Signal 2 to acquire full effector function. Signal 2 is normally transmitted by B7 family molecules like B7.1 or B7.2 on the surface of a functional antigen-presenting cell to a receptor molecule on the T cell called CD28. In some settings, though, CTLA-4 expression is up-regulated on the surface of a T cell; this often happens in tumors or in tumor-draining lymph nodes. CTLA-4 binds to B7 molecules with higher affinity than CD28 does, effectively highjacking Signal 2 and turning that T cell off. Anti-CTLA-4 monoclonal antibodies like Ipilimumab (Bristol Myers Squibb) and Tremilimumab (Medimmune) block this interaction, essentially allowing Signal 2 to proceed and T cells to be fully activated. This strategy has proven to be effective in metastatic melanoma, and Ipilimumab is FDA-approved in that setting. As might be expected from its MOA, administration of Ipilimumab is associated with immune-related adverse events (IRAEs), which typically include dermatitis, colitis, hepatitis, and others. IRAEs usually respond well to prompt treatment with immunosuppressive doses of corticosteroids, and treatment algorithms have been developed to assist with the management of those toxicities.
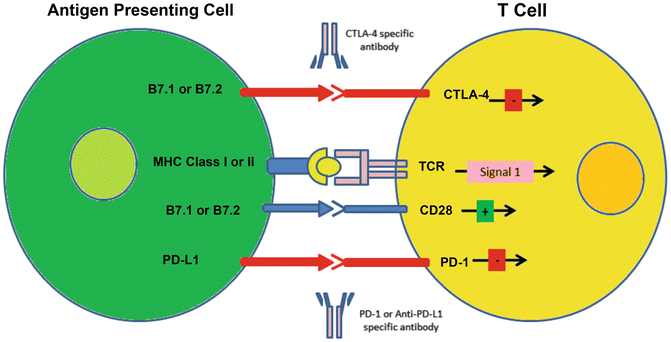

Fig. 9.2
Immune checkpoints: T cell activation requires both signal 1 (MHC + peptide/TCR) and signal 2 (B7.1 or B7.2/CD28). When a T cell up-regulates CTLA-4 (as in the tumor microenvironment), high-affinity binding of B7 molecules by CTLA-4 effectively highjacks signal 2. A monoclonal antibody directed against CTLA-4 (Ipilimumab or Tremilimumab) blocks that negative interaction, leading to T cell proliferation and effector function. The interaction between PD-L1 and PD-1 is also inhibitory, and can similarly be blocked with monoclonal antibodies directed against either PD-1 or PD-L1
Anti-CTLA-4 (Ipilimumab) in Prostate Cancer
Ipilimumab has been evaluated in a number of early phase studies in men with prostate cancer. These data were recently summarized by Slovin et al., and show that treatment is associated with a PSA response rate of approximately 15–20 %, and with few objective (radiographic) responses [48]. In several of these studies, a low dose of radiation therapy was tested in an effort to “release antigen” and potentiate an immune response. However, in the small dataset accumulated there was no evidence for such an effect; for example, the PSA decline rate in patients treated with a dose of 10 mg/kg of Ipilimumab was 12 % in the presence of radiation therapy (RT) versus 25 % without. Despite this relatively low PSA response rate, and little evidence that low-dose RT applied to a single lesion in men with metastatic disease augmented the response rate to Ipilimumab, a phase III trial combining RT + Ipilimumab was launched in men with mCRPC who had progressed on after treatment with docetaxel chemotherapy. This trial enrolled approximately 800 men and randomized them to a single low-dose treatment with RT alone versus RT followed by Ipilimumab at a dose of 10 mg/kg q 3 weeks × 4, followed by q 3-month maintenance for men who were not progressing. The primary outcome of this trial was overall survival, and results were recently reported. Disappointingly, the trial did not meet its primary overall survival (OS) endpoint with a median OS of 11.2 months in the Ipi group versus 10.0 months for placebo, The pre-specified secondary endpoint progression free survival (PFS) was met, with PFS = 4.0 in the Ipi group versus 3.1 in the placebo group (HR = .070, p < 0.001). Retrospective analyses of the results showed that men with more favorable disease characteristics (no visceral metastases, normal alkaline phosphatase, normal Hgb) appeared to possibly benefit from the treatment, although that kind of a post-hoc result clearly requires verification in a prospective trial. In that line, a second trial comparing Ipilimumab versus placebo was conducted in the pre-chemotherapy setting, results from that trial are still pending at this time. Taken together, these data suggest that Ipilimumab may have some activity in mCRPC, but that patients with more favorable characteristics, particularly those without visceral metastases might be more likely to benefit.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree