Fig. 3.1
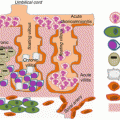
Under eubiotic environment the intestinal endothelial cells secrete mucins and cytokines, which conditions dendritic cells to promote an antiinflammatory cellular and mediators to dampen effector responses
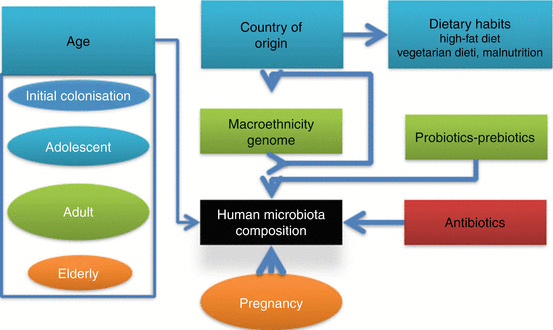
Fig. 3.2
Factors influencing the human microbiota composition
In the following sections, we will review some of the most recently published papers on the impact of diet on microbiota/microbiome structure, focusing on data obtained in humans.
3.2 Diet and Microbiota
The mutualistic relationship between the host and its microbiota leads to the production of beneficial microbial metabolites that contribute to the fitness of the host. The latter factor can influence the gut environment modifying the transit time, the pH, and, as regards the distal part, the richness in terms of carbohydrates, proteins, and fats related to food intake. These macronutrients are directly degraded by the host enzymes, while the degradation of plant structural polysaccharides, contained in the diet, is carried out by microbial enzymes encoded by specific microbial genes above all those of the colonic microbiota. Fermentation of these complex polysaccharides produces short-chain fatty acids such as butyrate, which represents one of the major energy sources for colonocytes, or propionate and acetate involved in gluconeogenesis and in lipogenesis respectively [11]. The dietary residues in the colon can represent a substrate for bacteria growth as well as the sloughed epithelial cells and mucin. The ability of bacteria to utilize different substrates may be crucial for the final microbiota composition, which is the final result of complex selection mechanisms of ingested bacteria.
Observational studies have shown that long-term diet influences the structure and activity of the trillions of microorganisms residing in the human gut, and it was also suggested that it could be possible to divide the human beings into three major groups according to their bacterial content.
Indeed, each of them is characterized by the dominance of Bacteroides, Prevotella, and Ruminococcus, respectively [12]. It is still unknown which environmental or genetic factors are involved in this clustering although it appears independent of nationality, sex, age, or other host properties. Wu et al. [13] described that dietary effects primarily distinguish the Prevotella enterotype (carbohydrate) from the Bacteroides enterotype (high protein and fat), and in this study a short diet intervention was insufficient for switching between the two groups. Moreover, it is important to underline that the enterotype hypothesis is a topic of debate. Some microbial groups exhibit high stability, but important quantitative fluctuation can be detected among bacteria that comprise the intestinal microbiota. These fluctuations move around an individual microbiota composition, and they can be due to variation of external environmental factors such as diet or drug treatments. When these species fluctuations do not exceed the limits that characterize a specific enterotype, but they remain at the borderline, no changes in enterotype can be detected.
This could be the reason of some contradictory reports. Indeed, recent data [14] described how even short-term intervention can modify gut microbiota composition: red meat based diets increase the prevalence of Alistipes, Bilophila, and Bacteroides, while decreasing the abundance of Firmicutes (plant polysaccharide metabolizers). The enterotype clusterization was not taken in consideration, and maybe this could justify the divergent results.
3.3 Microbiome, Macro Ethnicities, and Local Diets
A very interesting approach for evaluating the effect of diet on gut microbiota composition was adopted in a publication [15] in which the gut microbiota composition of children from a rural area in Africa (Burkina Faso) and Italian children were compared. The European subjects eat a typical Western diet (rich in proteins and fats), whereas the African children eat carbohydrate-rich diets with a very low supply of animal proteins. Xylanibacter, Prevotella, Butyrivibrio, and Treponema were distinctive of Burkina Faso children, whereas European children microbiota were characterized by the abundance of Firmicutes and Proteobacteria. The African children harbored microbiota in which it was possible to detect bacteria able to use xylan, xylose, and carboxymethylcellulose. It is possible to suppose that these polysaccharide-degrading bacteria are selected by the diet and are the responsible of short-chain fatty acids production using plant polysaccharides allowing the host to maximize the energy intake from the diet.
These results strongly indicate the important role of diet in shaping the microbial composition of the gut and revealed specific adaptations of gut microbiota communities to the lifestyles of the host. Confirmation of this founding is provided by a recent publication in which the gut microbiota composition of two different rural farming groups living in Tanzania (the Hadza population) was analyzed [16]. The gut microbiota of African voluntaries resulted in enriched Prevotella, Treponema, and unclassified Bacteroidetes. Interesting is the differences found comparing woman and men gut microbiota composition; indeed Treponema was more abundant in woman, whereas Eubacterium and Blautia were more represented in men. Women normally have a vegetarian diet, while men have the opportunity to eat meat and honey. Comparing the results of Hadza subjects with those obtained from the Italian control group, the total absence of Bifidobacterium was highlighted. Authors supposed that the postweaning in this population could explain this lack of bifidobacteria, while Western adult diet includes also meat, milk, and dairy products.
It is necessary to underline that Burkina Faso lifestyle differs from that of Tanzanian subjects overall in terms of diet; indeed around 70 % of Hadza diet is represented by plant foods (tubers, fruits, and vegetables) and the remaining 30 % by meat derived from hunting. Concerning Burkina Faso, children’s diet [15] consists mainly of cereals, legumes, and vegetables; therefore, the content of carbohydrate, fiber, and vegetable protein is very high. Comparison between these populations and the European subjects showed that microbial composition reflects differences between herbivorous and carnivorous mammals, and for this reason it became relevant, for human’s well-being, to try to modulate the composition of the bacterial population inhabiting the gastrointestinal tract. One of the key mechanisms to maintain gut health is the consumption of diet rich in nondigestible carbohydrates and poor in proteins and fats [17].
3.4 Gut Microbiota and Probiotics
The rationale of probiotic use is to introduce microorganisms with specific and beneficial functions in order to obtain positive effects on the health of the host. Indeed the definition of probiotic is “live microorganisms which, when administered in adequate amounts, confer a health benefit to the host” [2].
The first condition to satisfy is to administer living probiotic cells. We know that sonicates, particles of dead probiotics, can stimulate the immune system, yet only viable bacteria cells may be able to reproduce and exercise a longer beneficial effect on the bacterial communities of the gastrointestinal tract. To achieve this goal, it is essential that probiotic bacteria pass beyond the gastric and ileal tract and reach in a viable form the large intestine. The second important aspect to be evaluated is the administration of an effective dose. This is a very unsettled frontier. Gut’s pH, bile salt, and trilions resident microbiota outnumber low dose, short term supplementation of probiotics. All these factors play against the chances of an adequate impact on the microbial ecology of gut environment. Large doses, by hundred billions of viable cells for long periods of supplementation are required to provide beneficial effects.
The definition of the effective dose is not easily defined depending on several factors, and it depends on the strain-specific feature, the chemical and physical characteristics of the vector food, and the host-related factors as well as the desired target effect [20].
The guidelines of the French Agency for Food Safety dedicated to probiotics and prebiotics [21] suggest that the number of viable cells in the gut present after consumption of product containing probiotic must be more than or equal to 106 CFU/ml in the small intestine and 108 CFU/g in the colon, even if the same agency acknowledges that “the scientific basis for these statements is relatively weak.”
One of the more important concepts that need to be taken into consideration is that a probiotic must be a microorganism or a combination of microorganisms taxonomically well defined. The identification at species and strain level is very relevant for different reasons. The correct identification of a probiotic as a member of a recognized and well-known species corresponds to the evaluation of its safety. Indeed the bacterial species used as probiotic have a very long history of use in food, and this contributed to define them as safe for human consumption. On the other hand, the identification at strain level is relevant since the beneficial properties are strain specific that means the health benefit assigned to one strain is not necessarily applicable to another strain even belonging to the same species.
Adhesion to the intestinal mucosa is considered a prerequisite for bacterial colonization, and this event is important for the immune system modulation and for the antagonistic action of probiotic against pathogens. These adhesion properties can be mediated by secreted bacterial proteins that are able to interact directly with mucus (Figs. 3.3 and 3.4).

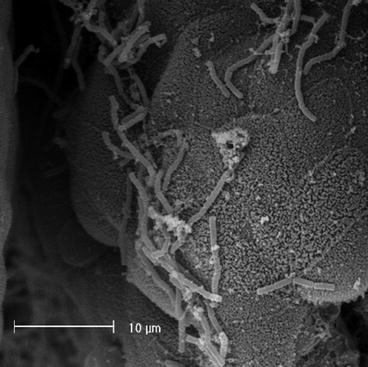

Fig. 3.3
Scanning electron microscopy picture of L. crispatus cells that adhere to intestinal mucus

Fig. 3.4
Scanning electron microscopy picture of L. gasseri cells adherent to intestinal epithelium
As already mentioned, probiotics have also important involvement concerning the immune response in enhancing signaling in host cells reducing the inflammatory response, delivering antiinflammatory molecules, and reducing the production of inflammatory substances [22–24]. Unfortunately, to demonstrate the positive effect of probiotic bacteria intake in healthy adults is extremely difficult. One of the problems to face is the stability, in healthy adults, of the complex composition of the gut microbiota which unlikely can be altered by the introduction of a single bacterial strain and even a combination of more strains. For this reason the ecological use of probiotic should be held when specific conditions of altered microbiota occur as a consequence of inflammatory diseases or antibiotic treatments. In these conditions there are strong evidences concerning the benefit of probiotic administration [25–27].
However, it has been described that probiotic strains can replace other indigenous strains even if administered to healthy individuals with a stable microbiota. In these cases the introduction of probiotic strains did not alter the overall microbiota composition, but it was possible to obtain its beneficial modulation. This replacement effect was demonstrated in a study [28] in which a L. paracasei strain was administered to seven healthy subjects; after 15 days of administration no statistically significant difference in the total amount of lactobacilli was found, but the probiotic strain represented 66.6 % of the total facultative heterofermentative colonies isolated from fecal stools. The probiotic strain in these cases replaced the lactobacilli originally present in the fecal samples of the treated volunteers. Another aspect in which the administration of probiotic to healthy subjects could be very interesting is the impact of probiotics on intestinal transit. This is an increasingly important measure of well-being because of the high incidence of constipation particularly in the elderly women and overall in pregnant women. The probiotic administration can represent an efficacious and safe treatment well suited for these groups of individuals. The administration of probiotics for 4 weeks to 20 young pregnant women with functional constipation improved the defecation frequency [29] that significantly increased from 3.1 at baseline to 6.7 at the end of the trial. Also some secondary effects normally correlated with constipation such as stool consistency, sensation of incomplete evacuation, or abdominal pain were reduced, and no side effects were reported.
The common cold very common especially in winter is responsible for many absences at school and from job and causes important economic losses. In a recent meta-analysis [30] in which ten studies were taken in consideration, the effect of probiotics on the prevention of the common cold was evaluated. The total number of participant was 2,894 participants, including 1,588 in the probiotic group and 1,306 in the control group. The relative risk used to evaluate the efficacy of probiotic intake was of 0.92 for the control group whereas 0.87 for the group receiving probiotics. The authors report that probiotics have a marginal effect on the prevention of the common cold, but it is important to underline that probiotics do not correspond to a medication, and no side effects are related to their administration as it sometimes happens for drug treatments.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree