Patient-related factors
Performance status
Nutritional status
History of previous neutropenic fever episodes increases risk
Risk of neutropenic fever in cycles 2–6 is 4 times higher if a neutropenic fever occurred during cycle 1 than if it did not [30]
Increasing number of co-morbidities increases risk
The odds for neutropenic fever increases with the number of co-morbidities by 27, 67, and 125 % for 1, 2, and 3 or more co-morbidities [48]
Underlying malignancy-related factors
Cancer diagnosis:
Acute leukaemia/myelodysplastic syndromes (highest risk) [58]
Soft tissue sarcomas, 26.8 %, 95 % CL 19.1–34.5 %
Non-Hodgkin’s lymphoma/myeloma, 26.0 %, 95 % CL 22.2–29.3 %
Germ cell carcinomas, 22.9 %, 95 % CL 16.6–29.1 %
Hodgkin’s lymphoma, 15.4 %, 95 % CL 6.6–24.2 %
Ovarian carcinoma, 12.1 %, 95 % CL 6.6–17.7 %
Lung cancers, 10.3 %, 95 % CL 9.8–10.7 %
Colorectal cancers, 5.5 %, 95 % CL 5.1–5.8 %
Head and neck carcinoma, 4.6 %, 95 % CL 1.0–8.2 %
Breast cancers, 4.4 %, 95 % CL 4.1–4.7 %
Prostate cancer, 1.0 %, 95 % CL 0.9–1.1 %
Cancer stage:
Status of the cancer during the time at risk for neutropenic fever syndromes:
Response to treatment
Complete responses are at lower risk [58]
Partial responses in solid tissue malignancies are at lower risk than partial responses in acute leukaemia
Treatment-related factors
Choice of cytotoxic regimen:
Higher risk regimens are those containing doxorubicin or epirubicin ≥90 mg/m2, cisplatin ≥100 mg/m2, ifosfamide ≥9 g/m2, cyclophosphamide ≥1 g/m2, etoposide ≥500 mg/m2, or cytarabine ≥1 g/m2 [84]
Dose-dense regimens such as CHOP-14 [83]
Dose intensity of the anticancer regimen
Degree and duration of monocytopenia (AMC <0.15 × 109 l) [78]
Administration of antimicrobial chemoprophylaxis may reduce risk in selected patients [30]
6.2 Neutropenia and Timing of Neutropenic Fevers
The relationship between fever, infection, and the state of neutropenia was described in the seminal work by Bodey and colleagues over 40 years ago [13, 14]. The risk of invasive infection is inversely related to the circulating absolute neutrophil count (ANC) [13, 22, 90] and increases as the ANC falls below 1.0 × 109 l and, in particular, as it falls below 0.5 × 109 l (OR 2.4, 95 % CI 1.3–4.5) [87]. Accordingly, severe neutropenia, as it is related to the risk of neutropenic fevers and documented invasive infections, is defined by an ANC below 0.5 × 109 l [22]. A relationship between the risk for neutropenic fevers and an absolute lymphocyte count (ALC) of <0.7 × 109 l at day 1 of cytotoxic therapy [8, 84] has also been observed. The ANC and ALC are readily available from most automated leukocyte differential counts that are performed in routine hospital and clinic laboratories.
The nadir of cytotoxic therapy-induced myelosuppression typically occurs at the end of the second week, between day 10 and 14, from the first day of cytotoxic therapy [21]. This is, coincidentally, the time of the maximum cytotoxic effect of the anticancer chemotherapies on the intestinal mucosa [20, 21, 65] and the time of maximal oral and gastrointestinal mucositis [9, 11, 21, 85, 93, 95]. Therefore, the median time of the first neutropenic fever is typically between day 10 and 14 of the chemotherapy cycle [21, 22] which corresponds to this time of maximal cytotoxic therapy-induced intestinal epithelial mucosal damage [20, 21] and is independent of the regimen [18, 74, 95, 110].
The majority of first neutropenic fevers tend to occur during the first of a multicycle regimen of systemic cytotoxic therapy [28, 30]. Moreover, a neutropenic fever episode occurring in cycle 1 tends to predispose to further episodes during subsequent cycles [30]. The risk is related to the type of cancer [48] and to the chemotherapeutic regimen [1, 108].
The majority of infections in febrile neutropenic patients are due to bacteria and opportunistic yeasts that normally colonise the cytotoxic therapy-induced damaged mucosal surfaces [10, 75, 88]. It is not surprising, therefore, that the microorganisms that are most often associated with invasive bloodstream infections in neutropenic patients are derived of the normal microflora of the periodontium (viridans [alpha haemolytic] group streptococci) and the gastrointestinal tract (facultatively anaerobic gram-negative members of the family Enterobacteriaceae [e.g. Escherichia coli, Klebsiella pneumoniae, or Enterobacter spp], Enterococcus spp. [often referred to as nonhaemolytic streptococci prior to genus and species identification], Staphylococcus spp. [including thermonuclease-positive S. aureus and thermonuclease-negative S. epidermidis], Candida spp. and less commonly obligate anaerobic gram-positive [Clostridium spp., Lactobacillus spp] and gram-negative [Bacteroides spp.] bacteria or a fermentative obligately aerobic gram-negative bacilli [Pseudomonas spp., Stenotrophomonas spp., or Acinetobacter spp.]).
The duration of severe neutropenia is different among different patient groups and the respective cytotoxic regimens. The expected median duration of an ANC <0.5 × 109 l among patients receiving remission-induction therapy with cytarabine and an anthracycline over 7 and 3 days, respectively, is of the order of 17 days [96] compared with 4 days for patients with solid tumours or lymphoma treated with intermittent cycles of chemotherapy [71]. The longer the duration of severe neutropenia, the greater the risk for opportunistic infection [13, 46].
6.3 Risks and Predictors for Neutropenic Fever
A number of factors have been observed to be associated with the risk for neutropenic fever syndromes. Of these, chemotherapy-induced neutropenia is perhaps the most important and is a common reason for anticancer treatment dose delays or dose reductions [89] that impacts upon the efficacy of the anticancer treatment regimen. These factors are listed in Table 6.1. Moreover, the degree of chemotherapy-induced oral and gastrointestinal mucositis is directly correlated with the risk of infection [94].
Various studies have identified risk factors for neutropenic fevers including older age (particularly ≥65 years), advanced underlying malignant disease, low baseline leukocyte counts, marrow myelophthisis, low serum albumin, anaemia, elevated lactate dehydrogenase, and co-morbid renal, cardiovascular, or hepatic conditions [82]. Such delays and reductions result in reduced dose intensity which, in turn, is linked to suboptimal anticancer treatment delivery [82, 83]. For example, in a 20-year follow-up to adjuvant CMF therapy for node-positive breast cancer, the overall survival of women who had received ≥85 % of the planned dose of CMF was 52 % compared to 32 %, 25 %, and 25 % for women who had received 65–84 %, <65 %, and 0 % (the untreated control group), respectively, of planned CMF therapy [15]. Patient outcomes are better when anticancer treatment delivery is optimal [17, 25].
A number of patient-driven circumstance-dependent predictors for neutropenic fevers have been identified including patient age (particularly 65 years or more) [40, 67, 102, 103], female sex [66], high body surface area [89], and pre-existing cardiovascular, renal, endocrine, or respiratory co-morbidities giving rise to poor performance status [66, 109] and poor nutritional status [52]. Disease-related predictors have included elevated lactate dehydrogenase (LDH) in lymphoreticular diseases [52], myelophthisis [52] with lymphopenia [8, 84], and advanced stage of the underlying malignancy [47, 58, 66, 103, 108]. Anticancer treatment-related predictors of neutropenic fevers have included administration of the planned dose intensity or dose density [67], administration of high-dose chemotherapy regimens [1, 83, 84, 92], and failure to administer haematopoietic growth factor support to patients receiving high-risk regimens [1, 102].
Different regimens carry varying risks for chemotherapy-induced neutropenia [1, 84, 92]. For example, prior to 1998, regimens based upon cyclophosphamide, methotrexate, and fluorouracil (CMF) were most frequently employed in the treatment of breast cancer [89]. Thereafter, anthracycline-based regimens became more common. The majority (70 %) of breast cancer patients receiving systemic chemotherapy now receive anthracycline-based regimens (such as FEC [fluorouracil, epirubicin, and cyclophosphamide]) or taxane plus anthracycline-based regimens (such as TAC [taxotere (docetaxel), doxorubicin (Adriamycin™), and cyclophosphamide]). The cycle length for such regimens is usually 21 days. The mean number of cycles is 7.9 ± 0.8 [83]. Neutropenic events (defined as neutropenia-related dose delays of ≥7 days, dose reductions of ≥15 %, or hospitalisations) have been more common among recipients of taxane-based regimens, followed by CMF-based regimens, and anthracycline-based regimens [89]. More neutropenic events occur among CMF recipients administered with increased dose density over 21 days rather than 28 days [62]. Neutropenic events occurring during cycle 1 of the treatment have tended to predict such events during the second and subsequent cycles [89]. Neutropenic fever syndromes have been uncommon among women receiving CMF-based adjuvant chemotherapy compared to those receiving anthracycline- or taxane-based chemotherapy regimens (none versus 5–6 %, respectively) [83]. Among patients treated with CHOP-like (cyclophosphamide, hydroxydaunorubicin [doxorubicin], vincristine [Oncovin™], and prednisone) regimens for non-Hodgkin’s lymphoma, grade 4 neutropenia over the course of 6–8 cycles may be expected in one in two patients, but the event rate for neutropenic fevers may be expected in up to 22 % [83]. Increasing the dose density of CHOP by reducing the time in between cycles from 21 to 14 days increases the likelihood that a toxicity-driven dose reduction will be required (from approximately one in three patients to up to one in two patients, respectively) [83]. The French ELYPSE study group characterised regimens at high risk for neutropenic fevers as those containing anthracyclines (doxorubicin or epirubicin) ≥90 mg/m2, cisplatin ≥100 mg/m2, ifosfamide ≥9 g/m2, cyclophosphamide ≥1 g/m2, etoposide ≥500 mg/m2, or cytarabine ≥100 mg/m2 per course [84]. Choice of chemotherapeutic regimen is a key driver of cytotoxic therapy-induced complications including grade 4 neutropenia, onset of neutropenic fever syndromes, and reductions in relative dose intensity and consequent impact upon survival.
6.4 Classification of Neutropenic Fever Syndromes
There are several neutropenic fever syndromes that have been described [18]. These consist of first neutropenic fevers, persistent neutropenic fevers, and recrudescent neutropenic fevers for a given neutropenic episode. The number and severity of recrudescent fevers depends upon the duration of neutropenia [46, 76]. Further, each fever may be classified as documented, microbiologically (based upon identification of a pathogen isolated from an infectious focus) or clinically (based upon identification of an infectious focus without a putative pathogen), or as unexplained [50]. At the time of presentation, it may not be possible to accurately classify the neutropenic fever syndrome until the results of investigations and cultures are known. For example, a bacteraemic patient who presents with no obvious inflammatory focus may be misclassified as an unexplained fever until the blood culture results become available.
The ultimate classification of the neutropenic fever syndrome will depend, to some extent, upon the rigour with which a clinical focus of infection is sought. At the time of presentation, between one-fifth and one-third of neutropenic fevers will prove to be bloodstream infections [44, 80, 104]. In one American study among first neutropenic fevers, bloodstream infections accounted for 23 %, unexplained fevers 8 %, and clinically documented infections 69 % [80]. Of the bloodstream infections, Staphylococcus spp. accounted for 19 %, viridans group streptococci 27 %, other gram-positive organisms 16 %, and gram-negative bacilli 37 %. Of the clinically documented infections, the majority (63 %) originated in the gastrointestinal tract (oral mucositis, oesophagus, and enterocolitis), 10 % originated in the skin and soft tissues (the majority of which were central venous access device related), 10 % originated in the lower respiratory tract, and 8 % from the urinary tract. In contrast, another trial from Europe classified the neutropenic fever syndrome as microbiologically documented bloodstream infections in 40 % of cases, non-bacteraemic microbiologically documented infections in 6 % of cases, unexplained fevers in 43 % of cases, and clinically documented infection in only 11 % of cases [23]. The major differences in these reports were in the proportions of infections that were bacteraemia and clinically documented oral and gastrointestinal mucositis. Despite the known relationship between cytotoxic therapy-induced mucosal damage, translocation, and invasive infections in neutropenic patients [12, 20, 94], there remain differences of opinion regarding the classification of sites of mucositis as infectious foci [85].
6.5 Is the Patient Febrile? Measurement of Body Temperature
An elevated body temperature may be the earliest and only sign of infection in the neutropenic patient [90]. Prompt initiation of empirical systemic antibacterial therapy is important to avoid progression to a sepsis syndrome and possibly death. Accordingly, the accurate and reliable clinical recognition of a febrile state in neutropenic patients is critical.
The origins of the definition of normal body temperature are somewhat obscure [68]. The work of Carl Wunderlich in 1868 suggested that a normal body temperature was 37 °C (98.6 °F) and that the upper limit of normal was 38 °C (100.4 °F), beyond which fever was defined [111]. Indeed, a survey of 270 medical professionals noted that the majority (75 %) believed that the normal body temperature was 37 °C (98.6 °F) [69]. However, a study of 148 healthy men and women at the University of Maryland demonstrated the mean of 700 baseline oral temperatures to be 36.8 ± 0.4 °C (98.2 ± 0.7 °F) with a range of 35.6 °C (96.0 °F) to 38.2 °C (100.8 °F). According to these observations, the upper limit of normal would be 38.2 °C (100.8 °F). The temperature of 37 °C accounted for only 8 % of all readings and fell outside of the 99.9 % confidence limit for the sample mean [70].
Given these considerations, a number of guidelines have been published to provide some direction regarding the definition of a febrile state in neutropenic cancer patients. The Infectious Diseases Society of America has defined a febrile neutropenic episode as a single oral temperature of >38.3 °C (101 °F) or a temperature of >38.0 °C (100.4 °F) sustained for >1 h [49]. Other international guidelines from North and South America, Europe, and Asia have provided similar definitions [6, 55, 64, 86, 101]. The Japan Febrile Neutropenia Study Group and the Asia-Pacific febrile neutropenia guidelines group have recommended that a single oral temperature of ≥38.0 °C or a single axillary temperature of ≥37.5 °C be accepted as the definition of a febrile state [101]. Based upon the observations pertaining to the range of normal temperatures from the University of Maryland, most North and South American and European guidelines have adopted the standard of a single oral temperature of ≥38.3 °C as the definition of pyrexia in the setting of neutropenic cancer patients.
The next important question is how body temperature is measured. Most medical facilities measure body temperature by oral, infrared tympanic membrane, axillary, or rectal thermometry as a surrogate of core body temperature as measured by standard pulmonary artery catheter (PAC) or in situ urinary bladder thermometry. An accurate measurement is desirable since the decision to initiate an aggressive protocol of neutropenic fever management may be based upon the difference of a half degree Celsius [34].
One study from St. George’s Hospital in London compared axillary chemical and infrared tympanic membrane thermometry to pulmonary artery catheter (PAC) thermometry for the estimation of core body temperature [36]. Based upon adjudication by an expert panel, false-negative rates resulting in possible delayed interventions of 15.3 and 21.1 % for axillary and tympanic thermometry, respectively, were observed. Similarly, false-positive rates possibly leading to unnecessary interventions of 28.8 and 37.8 % for axillary and tympanic thermometry, respectively, were observed. Whilst infrared tympanic membrane thermometry is non-invasive and convenient, the procedure is subject to inaccuracy due to observations obtained from the dependent ear [43], multiple user error [4], operator technique and equipment maintenance [39, 54, 77], and failure to remove cerumen in the external auditory canal [33]. Axillary temperatures have tended to be 0.2–0.4 °C higher than PAC thermometry [35, 36] thus overestimating patient temperature. Moreover, interventions such as warming blankets and haemofiltration have resulted in variances from PAC thermometric measurements by as much as 0.4 and 0.3 °C, respectively [36].
6.6 Risk for Serious Medical Complications Associated with the Neutropenic Fever Syndrome
Over 40 years ago, it was recognised that the population of neutropenic patients is very heterogeneous [13, 98, 99]. Neutropenic cancer patients are not only at risk of infection; they are at risk for a variety of medical complications of other treatments, other co-morbid diseases, and cancer-related problems that may lead to cardiorespiratory failure or bleeding [97]. The identification of co-morbidities among febrile neutropenic patients is linked to in-hospital mortality risk and cost of hospitalisation [60]. Early studies at the Dana-Farber Cancer Institute noted that on the average about one in five febrile neutropenic cancer patients would develop such problems; however, the risks for serious medical complications were about one in three for those who were inpatients or who had uncontrolled cancer at the time of the neutropenic fever and even higher, one in two, for those with coexisting active co-morbidities [98]. For those febrile neutropenic patients without these characteristics, a group that accounted for the majority of patients at risk of infection, the complication rate was only 2 % [98]. These observations suggested that these characteristics might be useful for developing a predictive rule to reliably identify a subgroup of patients for whom a different approach with transition to outpatient management may be feasible and safe [99, 100].
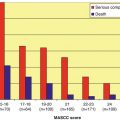
The Multinational Association for Supportive Care in Cancer (MASCC) has developed [58] and validated [105] a risk-index scoring system based upon characteristics easily identifiable at the onset of the episode predicting the development of potentially serious medical complications (shown in Table 6.2) during the neutropenic fever syndrome that would require hospitalisation or prolong hospitalisation for management. This risk-index score is presented in Table 6.3. A risk-index score of ≥21 in the initial development of the model predicted a group of patients at “low risk” for the kinds of medical complications shown in Table 6.2 with a sensitivity, specificity, and positive and negative predictive values of 71, 68, 91, and 36 %, respectively, with a misclassification rate of 30 % [58].
Table 6.2
Neutropenic fever syndrome-associated medical complications considered serious
Medical complications |
|---|
Hypotension (defined by a systolic blood pressure less than 90 mmHg or by the need for vasopressor support to maintain blood pressure) |
Respiratory failure (defined by an arterial oxygen pressure less than 60 mmHg whilst breathing room air or by the need for mechanical ventilation) |
Admission to a critical care service |
Disseminated intravascular coagulation |
Presence of confusion, delirium, or altered mental state |
Development of congestive cardiac failure documented by chest imaging and that requires treatment |
Bleeding diathesis sufficient to require blood cell transfusion |
Arrhythmia or ECG changes requiring treatment |
Renal failure sufficient to require investigation and/or treatment with IV fluids, dialysis, or any other intervention |
Other complications judged serious and clinically significant by the medical care team |
Table 6.3
The Multinational Association for Supportive Care in Cancer (MASSC) index score used to predict the likelihood of serious medical complications in “low-risk” versus “high-risk” febrile neutropenic cancer patientsa
Prognostic factor | Weight |
|---|---|
Burden of the neutropenic fever syndrome: no symptoms or only mild symptoms | 5 |
No hypotension (systolic BP >90 mmHg) | 5 |
No chronic obstructive lung disease | 4 |
Solid tissue malignancy or haematological malignancy without previous history of invasive fungal infection | 3 |
No dehydration that requires administration of parenteral fluids | 3 |
Burden of the neutropenic fever syndrome: moderate symptoms | 3 |
Outpatient status at the time of onset of the neutropenic fever syndrome | 3 |
Age <60 years | 2 |
In a prospectively conducted additional validation study, the MASCC risk-index score correctly classified low-risk and high-risk patients in 98.3 and 86.3 % of cases, respectively, giving a sensitivity, specificity, and positive and negative predictive values of 95, 95, 98.3, and 86.4 %, respectively [105]. The model was further refined by the reclassification of patients with “complicated” infections (defined by presence of a visceral site of infection, sepsis syndrome, a non-necrotising skin or soft tissue infection [SSTI] of >5 cm diameter, a necrotising SSTI of any size, or oral mucositis [WHO grade >2]) as high risk for serious medical complications. This had the effect of increasing the sensitivity and negative predictive value to 100 % each among febrile neutropenic patients with a MASCC index score of ≥21 [31]. Table 6.4 details a review of the published performance of the MASCC risk-index scoring system for predicting patients at low risk for serious medical complications. The pooled sensitivity, specificity, and positive and negative predictive values were 85, 68, 83, and 68 %, respectively. The rate of misclassification was one in five (20 %, 95 % CI 10–29 %).
Table 6.4
Review of clinical trials examining the performance of the Multinational Association for the Supportive Care in Cancer (MASCC) risk-index score for identifying febrile neutropenic patients at low risk for serious medical complications
Reference | True positive | False positive | False negative | True negative | SENS | SPEC | PPV | NPV | Misclassification rate |
|---|---|---|---|---|---|---|---|---|---|
Klastersky et al. [58] | 221 | 22 | 90 | 50 | 0.71 | 0.69 | 0.91 | 0.36 | 0.29 |
Uys et al. [105] | 57 | 1 | 3 | 19 | 0.95 | 0.95 | 0.98 | 0.86 | 0.05 |
Cherif et al. [24]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|