1
Introduction
Type 2 diabetes mellitus (T2DM) is a challenging epidemic disease affecting 12% of the middle-aged population [ ]. Its global incidence is increasing rapidly and dramatically, driven by an obesogenic environment that favors easier access to hypercaloric foods and more sedentary behavior. T2DM is associated with overwhelming health consequences, affecting large and small vessels and leading to nephropathy and impaired renal function, peripheral neuropathy and amputations, and retinopathy and vision loss, as well as life-threatening macrovascular disease [ ]. Consequently, it has become one of the major threats to public health worldwide. The problem is magnified by the fact that up to half of patients with T2DM are unaware that they have the disease [ ].
The pulmonary function is not considered a “classic” target organ in T2DM. However, because of its high vascularity and abundance of collagen and elastin fibers, the lung parenchyma is potentially sensitive to the deleterious effects associated with chronic hyperglycemia [ ]. Thus, there is good reason to believe that the same histologic and physiologic alterations that account for complications in other systems also affect pulmonary function [ ]. Although to date such alterations have had little clinical relevance, it should be noted that chronic declines in spirometric measurements such as forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) are independent predictors of cardiovascular and all-cause mortality in T2DM [ ]. In addition, basic research and epidemiological and clinical data also support the notion that T2DM has a deleterious effect on breathing during sleep, making it an independent risk factor for severe nocturnal hypoxemia [ ].
2
Baseline spirometric values in patients with T2DM
Multiple cross-sectional studies have repeatedly and systematically demonstrated that adult subjects with T2DM have lower FEV1 and FVC values than the population without T2DM ( Table 13.1 ). This decrease ranges from 8% to 10% of the predicted value for both parameters. Among the larger epidemiological studies that have published their results are the Fremantle Diabetes Study [ ], the Framingham Heart Study [ ], the British Women Heart and Health Study [ ], the Normative Aging Study [ ], the Atherosclerosis Risk in Communities (ARIC) Study [ ], and the Strong Heart Study [ ]. While it is true that most studies have been conducted in North America and Europe, there is corroborating evidence that T2DM is also associated with impaired lung function in Asian patients [ ]. Most of the available reports have considered numerous confounding factors, such as age, sex, body mass index (BMI), smoking history, and duration of diabetes. Regarding this latter factor, Barrett-Connor et al. observed that the decline in spirometric measurements was greater in patients with a duration of the disease of at least 10 years [ ]. Furthermore, it is repeatedly observed how higher fasting plasma glucose (FPG) and glycosylated hemoglobin values inversely correlate with lower FEV1 and FVC values [ , , ]. In other words, if you are a subject with T2DM, the worse your metabolic control the more reduced your FEV1 and FVC values will be. Although lung diffusing capacity (DLCO) has been assessed in fewer studies, there is no doubt that T2DM also has a negative impact on it [ ]. This effect is more evident with exercise and in patients that combine diabetes and obesity and is around of 10%–25% reduction compared with general population [ ].
| Author | Year | Sample | N | T2D versus non-T2D | ||
|---|---|---|---|---|---|---|
| FEV1 | FVC | DLCO | ||||
| Barrett-Connor and Frette [ ] | 1996 | Rancho Bernardo Study | 1239 | ↓ a | ↓ a | – |
| Davis et al. [ ] | 2000 | Fremantle Diabetes Study | 495 | ↓ | ↓ | – |
| Lange et al. [ ] | 2002 | Copenhagen City Heart Study | 12,062 | ↓ | ↓ | – |
| Walter et al. [ ] | 2003 | Framingham Heart Study | 3254 | ↓ | ↓ | – |
| Lawlor et al. [ ] | 2004 | British Women Heart and Health Study | 3911 | ↓ | ↓ | – |
| Litonjua et al. [ ] | 2005 | Normative Aging Study | 704 | ↓ b | ↓ b | – |
| Chance et al. [ ] | 2008 | Type 2 diabetic patients and Nonsmokers | 114 | ↔ | ↓ | ↓ c |
| Yeh et al. [ ] | 2008 | Atherosclerosis Risk in Communities Study | 11,262 | ↓ | ↓ | – |
| Oda and Kawai [ ] | 2009 | Japanese men and women | 2608 | – | ↓ | – |
| Oda and Kawai [ ] | 2009 | Japanese men and women | 1353 | – | ↓ | – |
| Klein et al. [ ] | 2012 | Clinical setting study | 4164 | ↓ | ↓ | ↓ |
a Association only noted in those with diabetes duration more than 10 years.
b FEV1 and FVC lower in the ever smokers but not in never smokers.
c Decrease in DLCO only noted with exercise and not at rest.
Beyond the cross-sectional studies that have highlighted the independent association between T2DM and lung function impairment, data from longitudinal studies are also available. In this regard, the results are not always concordant. Thus, in the Fremantle Diabetes Study and the ARIC Study, after 7 and 3 years of follow-up, respectively, a greater decrease in FVC was observed in subjects with T2DM than in the control population [ , ]. In these studies, duration of diabetes and poor glycemic control were directly associated with the degree of lung function deterioration over time [ , ]. Furthermore, the data obtained in these studies allow to estimate that lung function begins to deteriorate about 3 years before the diagnosis of hyperglycemia [ ]. However, data from the Normative Aging Study and the Copenhagen City Heart Study revealed no differences in longitudinal trajectories of lung function between participants with and without T2DM [ , ]. Taken together, cross-sectional and longitudinal data indicate that patients with T2DM may have lower FEV1, FVC, and DLCO values than nondiabetic subjects.
Recently, also patients with type 1 diabetes have showed lower spirometric values in comparison to the control group, together with a higher prevalence of FEV1 <80% and restrictive ventilatory pattern [ ].
3
What we know about mechanisms leading to pulmonary dysfunction in T2DM?
3.1
Which are the well-known mechanisms of pulmonary dysfunction in type 2 diabetes?
The etiopathogenic mechanisms by which T2DM exerts its deleterious effect on lung function are diverse, such as insulin resistance (IR), chronic inflammation, decreased muscle strength, nonenzymatic glycosylation of lung proteins, microangiopathy, and autonomic neuropathy. It must be considered that each one of them explains only part of the picture and that they can manifest simultaneously in the same patient [ ] ( Fig. 13.1 ) .
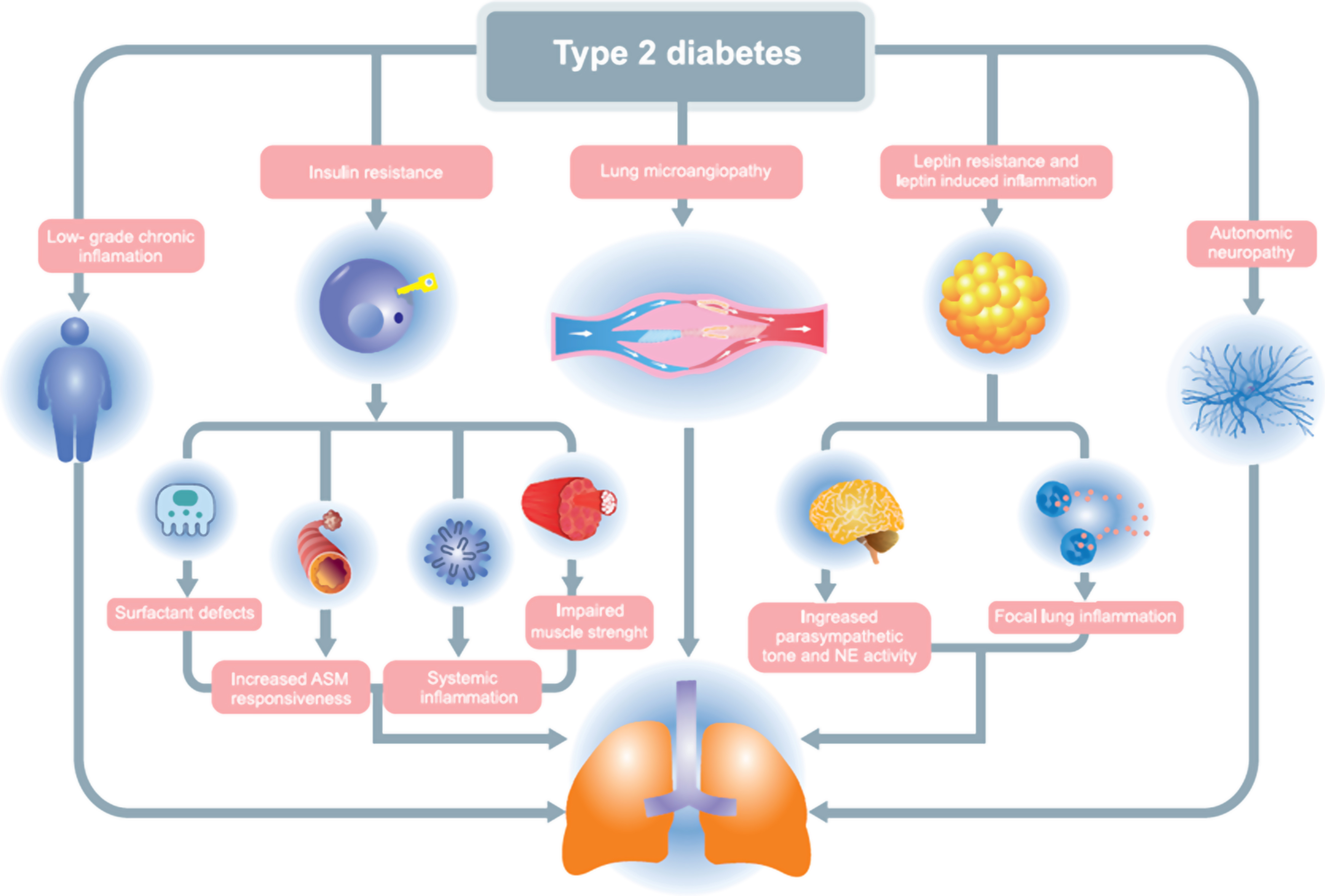
It is well known that IR is one of the main underlying mechanisms leading to T2DM. In morbidly obese women without diabetes, IR has been established as an independent determinant of airway resistance, contributing to the variation in FEV1, peak expiratory flow (FEF25-75), and FVC [ ]. In addition, IR has also been shown to be an independent predictor of expiratory reserve volume [ ]. Thus, metabolic pathways related to insulin resistance appears to be crucial in initiating pulmonary abnormalities in patients with T2DM and point to the lung as a new target of the metabolic syndrome.
A possible interaction between systemic inflammation, also inherent in the pathophysiology of DM2, and lung function has been proposed. For example, inflammation mediated by insulin resistance offers a biological explanation for its links to the incidence of asthma-like symptoms among adults [ ]. In this regard, both pharmacological inhibition with etanercept and genetic inhibition of tumor necrosis factor (TNF)-α (TNF-α R1KO) significantly reduce inflammatory cell infiltration, activates the proapoptotic pathway, and induce tight junction alteration in an animal model [ ]. Furthermore, in nonsmoking women with obesity without prior respiratory disease, soluble TNF-α 1 receptor (TNF-R1) independently contributes to decreased FEV1 and FVC spirometric values [ ]. Similarly, in T2DM patients without lung disease, elevated levels of inflammatory markers are associated with impaired lung function in those with an HbA1c ≥ 7.0% [ ]. These findings strongly suggest that T2DM-related inflammatory pathways are another early pathogenic factor involved in lung dysfunction, with a deleterious effect that can be appreciated before the development of clinical respiratory disease.
Damage to the pulmonary microcirculation, the major microvascular bed in humans, is related to glycemic control, reflects the degree of systemic microangiopathy, and can progress even after achieving adequate glycemic control [ ]. The large vascular and ventilatory reserve in humans manages to compensate for the initial loss of lung parenchymal function, so diabetic pulmonary microangiopathy initially causes underdiagnosed lung dysfunction for long periods. As a manifestation of this pulmonary microangiopathy, decreases in pulmonary DLCO have been reported, which correlate with the findings in the ophthalmoscopic examination and the rate of protein excretion in 24 h [ ].
In T2DM, changes at the muscle level are characterized by structural changes such as muscle atrophy, increased lipid deposition, decreased mitochondria concentration, and transformation of muscle fibers [ ]. All these changes favor the decrease in muscle strength observed in patients with diabetes and provokes a negative effect on contractile function and muscle force generation [ ]. Thus, in the Berlin Aging Study II, muscle mass together with abdominal obesity proved to be the most important factor influencing lung function in subjects with T2DM and metabolic syndrome [ ].
Finally, diabetic autonomic neuropathy (DAN) has also been associated with a higher prevalence of lung dysfunction [ ]. Thus, patients with T2DM and DAN showed reduced maximal inspiratory static pressure (PI max ), with a negative correlation between resting muscle strength and heart rate (HR) and duration of diabetes [ ]. Similarly, in adolescents with type 1 diabetes, the presence of cardiovascular autonomic neuropathy (CAP) assessed by heart rate variability was associated with impaired lung function tests, primarily significantly lower FVC, FEV1, and flow velocity values expiratory rate [ ].
3.2
Other contributors to pulmonary dysfunction in type 2 diabetes
Three more potential mechanisms related with the deleterious impact of T2DM in pulmonary function merits to be commented: leptin resistance, defects in the bronchiolar surfactant layer, and deficit in GLP-1 concentrations. Leptin acts on the bronchial tree inhibiting parasympathetic signaling in smooth muscle cells and favoring a physiological increase in bronchial diameter [ ]. However, in T2DM, leptin resistance is a common disorder that leads to increased parasympathetic tone, which in turn will cause bronchoconstriction [ ]. Leptin resistance has also been linked to hyperactivity of neutrophil elastase, an enzyme that can break down proteins in lung tissue and lead to lung disorders [ ]. Thus, in children and adolescents with obesity, a pathology in which resistance to the action of leptin has also been described, a negative correlation has been observed between leptin concentrations and FEV1, reinforcing the idea that the lack of action of this adipokine is a key factor in the development of a restrictive lung pattern [ ].Nonenzymatic glycosylation of lung connective tissue proteins, especially collagen but also elastin and fibronectin, may favor the restrictive pattern observed in patients with T2DM. In fact, nonenzymatically glycosylated collagen has low physiological turnover rates, is stiffer, and is also more resistant to collagenase and pepsin digestion than nonglycosylated collagen [ ]. In addition, the binding of RAGE activates pathophysiological cascades that lead to pulmonary endothelial cell dysfunction and promote proinflammatory effects and cell apoptosis [ ]. Finally, since the soluble receptor for AGE (sRAGE) blocks the action of AGE, the decrease in its serum concentration is associated with a greater progression of airflow limitations over time [ ].
Defects in the bronchiolar surfactant layer, which is involved in maintaining airway stability and diameter and preventing lung collapse, also contribute to impaired lung function in T2DM. Thus, surfactant protein levels in peripheral blood, which reflect alveolar-capillary membrane damage, have been associated with impaired glucose tolerance and insulin resistance [ ]. In addition, the glucagon-like peptide 1 (GLP-1) receptor has been found in significant amounts in the lung and most importantly, experimental studies have shown that GLP-1 plays a role in stimulating surfactant production by type 2 pneumocytes [ , ]. Therefore, the underlying deficit in GLP-1 concentrations that exists in T2DM could also increase the airway resistance seen in these patients.
4
Histopathological changes in lung parenchyma in T2DM
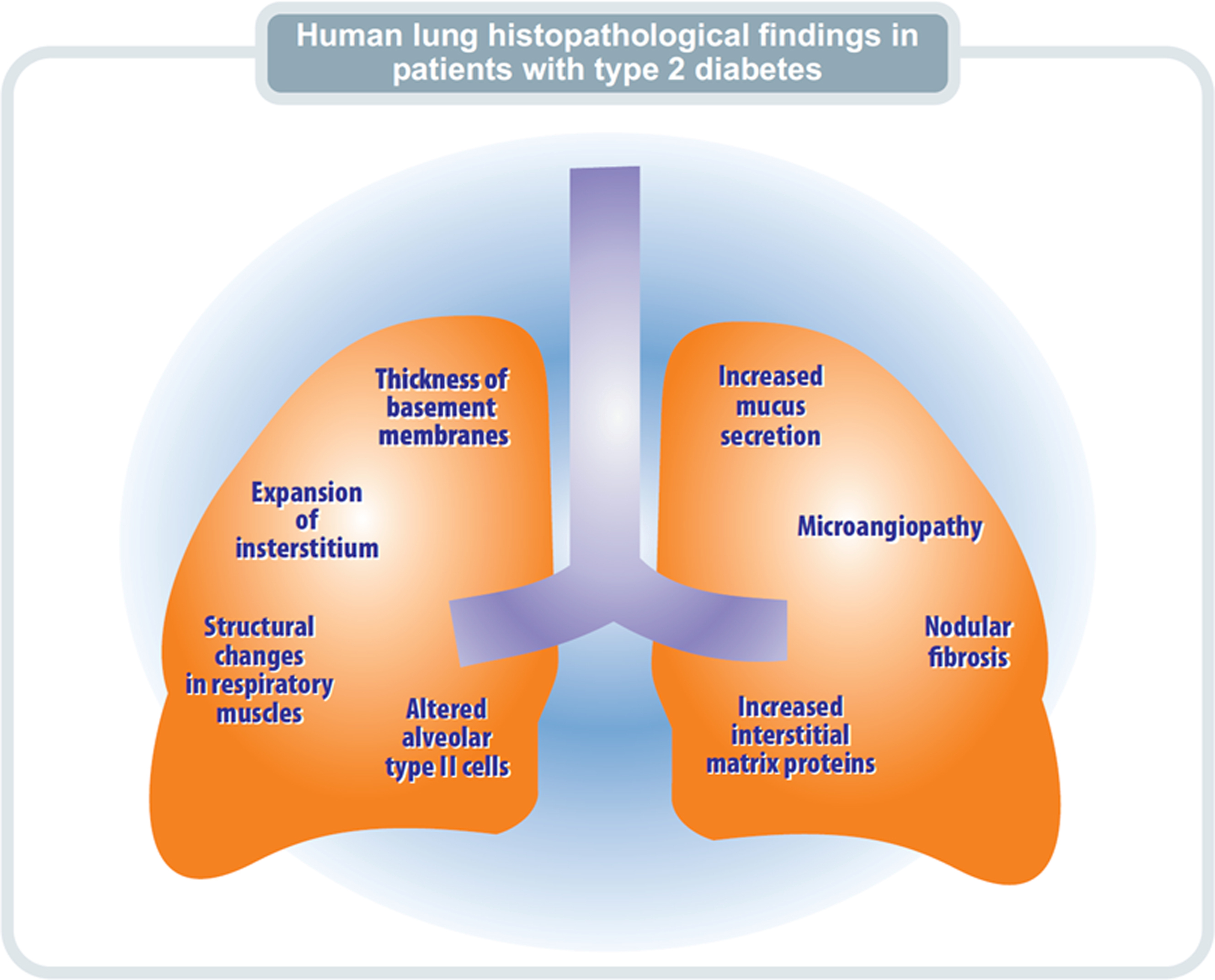
Histopathological changes at the lung level in T2DM are homogeneous throughout the parenchyma and include thickening of the alveolar epithelium and the basal lamina of the pulmonary capillaries, decreased alveolar space, a higher degree of fibrosis, centrilobular emphysema, and pulmonary microangiopathy, as well as variations in mucosal secretion [ ] ( Fig. 13.2 ) .