Category
Cytology diagnostic category
Malignancy risk (%)
I
Nondiagnostic or unsatisfactory
1–4
II
Benign
0–3
III
AUS/FLUS
5–15
IV
FN/SFN or SHCN
15–30
V
Suspicious for malignancy
60–75
VI
Malignant
97–99
Preoperative Evaluation
Ultrasound Evaluation
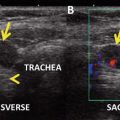
High-resolution ultrasound is generally considered to be the first-line imaging modality to assess the primary tumor and identify lymph node metastases at the initial evaluation of a suspected thyroid malignancy [1]. Ultrasound is widely available and inexpensive, provides detailed high-resolution anatomic information, avoids ionizing radiation, and facilitates ultrasound-guided FNA of suspicious lesions [4]. Ultrasound evaluation of bilateral cervical lymph node compartments (levels I–VI) should be performed routinely in the evaluation of patients with lesions suspicious for thyroid malignancy to guide a complete resection of the primary tumor as well as a compartment-oriented dissection of affected lymph node basins as necessary [1]. This will also facilitate patient counseling regarding surgical risks, since the addition of central or lateral neck dissection to total thyroidectomy is accompanied by a different or increased morbidity risk profile.
There have been many studies examining the utility of US to predict the risk of malignancy in patients with indeterminate FNA cytology, and several findings are commonly used to help guide preoperative surgical planning [5, 6]. Hypoechogenicity, increased nodular vascularity, irregular margins, the presence of microcalcifications, and taller-than-wide shape all correlate with an increased risk of malignancy [3]. These US findings may be used in a supplementary fashion to predict increased malignant potential in cytologically indeterminate nodules [5, 6].
Thyroid cancer lymph node metastases are common, especially in the central compartment, and are reported to occur in 12–81 % of patients with PTC overall and in a smaller proportion of patients with other histotypes (i.e., follicular thyroid cancer and Hürthle cell carcinoma) [7–9]. Lymph node metastases apparent preoperatively or intraoperatively (cN1) can be present in approximately 21–35 % of patients with differentiated thyroid cancer [10–12]. Microscopically positive lymph node metastases are far more prevalent, occurring in 38–62 % of patients with clinically negative (cN0) preoperative and intraoperative nodal assessments [13–15]. Not all lymph node metastases are the same in terms of their implications for recurrence and mortality. Macroscopic lymph node metastases (gross nodal disease) in patients with PTC are associated with higher recurrence rates, and an increased mortality rate has been observed in older patients with lymph node metastases [16–18]. In contrast, microscopic lymph node metastases do not affect patient survival and are associated with much lower rates of recurrence [19–21]. Clearly, the prognostic importance of nodal disease in PTC seems to be centered on detection and treatment of gross lymph node metastases. Despite occult lymph node micrometastases being found in the central compartment of the neck in 38–80 % of patients with PTC, the median rate of recurrence for patients with clinically node-negative disease is 2 %, whether or not a central neck dissection (CND) is performed [22, 23]. This finding suggests that most micrometastases remain dormant and infrequently evolve into clinically significant disease or alternatively that radioiodine ablation is adequate for the treatment of micrometastatic disease.
Ultrasound features predictive of malignant lymph node involvement include size greater than 1 cm, round shape, loss of hilum, cystic appearance, the presence of punctate calcification, and peripheral hypervascularity [1]. The sensitivity of US in detecting abnormal lymph nodes ranges from 25 to 60 % for the central neck and from 70 to 95 % for the lateral neck [1]. The sensitivity of US of the central neck is lower because the presence of the thyroid gland may prevent small central neck nodes from being detected. The specificity of US in detecting lymph nodes affected by metastatic PTC is high, ranging from 80 to 95 % in both the central neck and the lateral neck [24].
Ultrasound interrogation of the central and lateral neck lymph nodes may inform the clinician in the planning of surgery for cytologically indeterminate nodules. The discovery of lymph node metastases in this setting affords the opportunity to move forward with complete initial resection of all tumor tissue. Patients found to have abnormal central or lateral neck lymph nodes on US should undergo FNA biopsy of at least one of these nodes. The finding of cells of thyroid origin within lymph nodes or the presence of an elevated Tg in the needle washout, (taking into account the fact that serum Tg is normal or high because of the presence of the thyroid gland) confirms the diagnosis of metastatic thyroid cancer and may significantly alter surgical management in the patient with indeterminate thyroid cytology.
Molecular Marker Testing
Because about 25 % of thyroid nodules are “indeterminate” on FNA, a large number of diagnostic surgeries are performed every year in the United States and result in morbidity and higher health-care costs. Moreover, patients who are ultimately found to have malignant tumors, but who had indeterminate or suspicious FNA lesions, may require a completion thyroidectomy, especially if postoperative radioiodine is being considered. This challenge has led many scientists to investigate the utility of molecular markers in predicting the actual pathology of cytologically indeterminate thyroid nodules, in order to improve the sensitivity and specificity of FNA cytology. Owing to the overlap between follicular adenomas and follicular thyroid cancer, as well as the relatively low prevalence of known mutations in indeterminate FNA lesions, only a few molecular diagnostic tests with their own limitations have become commercially available.
Diagnostic molecular testing can detect mutations and translocations commonly seen in differentiated thyroid cancer including BRAF, RAS, RET/PTC, and PAX8/PPARγ and can be applied to FNA specimens. Another test, the gene expression classifier, analyzes the expression of over 140 genes and has a high negative predictive value if the result is benign. Recent large prospective studies have confirmed the ability of genetic markers (BRAF, RAS, RET/PTC, and PAX8/PPARγ), the gene expression classifier, and protein markers (galectin-3) to improve the preoperative diagnostic accuracy for patients with indeterminate thyroid nodules [25–27] and may become a more commonly utilized component of FNA evaluation in the future (Table 3.2). In addition, reverse transcription-polymerase chain reaction to detect thyroglobulin mRNA and thyrotropin-receptor mRNA from a lymph node is accurate for diagnosing metastatic thyroid cancer [28]. The current ATA management guidelines state that the use of molecular markers might be considered for specific patients with indeterminate thyroid nodules, rather than proceeding to surgery [3]. However, their utility in guiding preoperative surgical decision-making is yet to be proven. In addition, a recent study indicates these tests may be overused in patients for whom the results would not change surgical management [29].
Management
For patients with lesions suspicious for PTC or diagnostic of PTC (Bethesda category V or VI), total thyroidectomy should be performed and compartment-oriented neck dissection reserved for clinically apparent lymph node metastases to the central and lateral neck compartments [3]. CND plays an important role in the management of clinically apparent nodal disease. Completeness of surgical resection is an important determinant of outcome in patients with gross lymph node metastases, as residual metastatic lymph nodes represent the most common reason for disease persistence or recurrence, therefore influencing prognosis [3].
An area of major controversy in the management of DTC is whether patients with clinically negative lymph nodes (cN0) should undergo elective central neck dissection. This controversy largely came about following the 2006 ATA guidelines , which stated under recommendation 27, “Routine central compartment (level VI) neck dissection should be considered for patients with PTC” [30]. The strength of the recommendation was given a rating of B, indicating it was based on fair evidence that CND may improve health outcomes. At the same time, a European consensus statement on elective CND was endorsed by the European Thyroid Association and read: “there is no evidence that elective CND improves recurrence or mortality rates, but it does allow an accurate staging of the disease that may guide subsequent treatment and follow-up” [31]. In 2009, the revised ATA guidelines were published with a modification in the recommendation for CND. Recommendation 27B was modified to read “prophylactic CND (ipsilateral or bilateral) may be performed in patients with PTC with clinically uninvolved central neck lymph nodes, especially for advanced primary tumors (T3 or T4).” The strength of the recommendation was lowered to C, meaning that this was based on expert opinion [3]. In the new 2015 ATA guidelines [32], this recommendation is “weak, based on low quality evidence.” On the other hand, the 2009 and 2015 ATA guidelines state: “Thyroidectomy without prophylactic central neck dissection may be appropriate for small (T1 or T2), noninvasive, clinically node-negative PTC (cN0).”
This controversy is further compounded by fact that no randomized controlled trial exists to evaluate prophylactic CND for the treatment of DTC. A feasibility study completed by the ATA estimated that a randomized trial would require 5840 patients and would cost approximately $15 million [33]. The controversy over whether to perform prophylactic CND also relates to the differing interpretations of existing data. Nevertheless, a meta-analysis involving 1,264 patients found no significant difference in locoregional recurrence rates overall (2 % vs. 3.9 %) or within the central (1.9 % vs. 1.7 %) or lateral (3.7 % vs. 3.8 %) neck compartment with or without prophylactic CND [34].
Those who advocate prophylactic CND do so for its theoretical potential benefits, including reduced rates of recurrence [35, 36], lower postoperative serum Tg levels [14, 35], accurate staging to help modify indication for radioactive iodine ablation and dosing [37–40], and reduced reoperation in the central neck with its potential for higher morbidity [14, 19, 20, 41]. Those who oppose prophylactic CND do so because there is no proven oncologic benefit, primarily in terms of disease-specific mortality, and there is concern for an increased risk of recurrent laryngeal nerve injury and hypoparathyroidism [13, 38, 41–43].
Prophylactic CND for Small-Volume Microscopic Lymph Node Metastasis (cN0)
Small-volume microscopic LN metastases, not apparent on preoperative US exam, appear to be present in up to 80 % of patients diagnosed with papillary thyroid microcarcinomas. The locoregional recurrence rates in treated patients range from 2 to 6 %, regardless of the extent of lymph node dissection and whether or not radioactive iodine was given as adjuvant therapy after surgical resection [9].
Patients with macroscopic PTC (primary tumor >1 cm) have rates of microscopic nodal disease in up to 62 % of cN0 central neck compartments even though recurrence rates are only 1–6 % if CND was not performed [13, 14]. It appears that both microscopic and macroscopic PTCs are often associated with subclinical microscopic lymph node metastases that usually do not progress and become clinically relevant even if untreated [9]. A recent retrospective study by Bardet et al. [44] has shown that patients referred for radioactive iodine ablation between 2006 and 2011 with small lymph node metastasis (0.2 to <1 cm), and not micrometastases as defined by Randolph et al. [9], presented an intermediate outcome for persistence/recurrence between that observed in pN0-pNx patients and pN1 macroscopic patients.
Moreover, many researchers have reported the identification of various factors (i.e., larger tumors, extrathyroidal extension, and aggressive histological subtypes) that might favor prophylactic CND [45]. However, some of these factors such as extrathyroidal extension and aggressive histological subtypes cannot help in the decision to perform prophylactic CND because they are determined primarily by postsurgical histopathology.
Molecular Markers as a Guide for Surgical Extent
The presence of a BRAF V600E mutation has been associated in many studies with the aggressiveness of PTC (extrathyroidal invasion, lymph node metastasis, and advanced stage) and also with disease-specific mortality when associated with other aggressive features, such as extrathyroidal extension [46, 47]. However, BRAF V600E mutation analysis offers a limited positive predictive value (28 %) for disease recurrence [48], therefore suggesting that preoperative knowledge of BRAF V600E mutation status in the primary tumor should not impact on the decision for prophylactic CND.
RAS mutations are associated with a follicular pattern of thyroid neoplasms and have also been reported in poorly differentiated PTC [49]. The clinical significance of RAS mutations in thyroid cancer is controversial; some reports show that RAS mutations are associated with tumor aggressive phenotypes and poor prognosis [50, 51], while others could not confirm this association [52]. Similarly, RET/PTC rearrangements are associated in some reports with lymph node metastasis and extrathyroidal extension [53] and with a better prognosis in other studies [54, 55]. PAX8/PPARγ rearrangements have been associated with multifocality of the tumors and vascular invasion, conferring an invasive potential [56–58]. Others have found that PAX8/PPARγ rearrangement in thyroid nodules predicts follicular-pattern carcinomas, in particular the encapsulated follicular variant of PTC [59]. Despite this, the consistent detection of PAX8/PPARγ rearrangements in benign tumors hinders its value as a diagnostic and prognostic molecular marker [60]. In the above-presented case, the patient underwent molecular analysis for BRAF V600E, RAS, RET/PTC, and PAX8/PPARγ, all of which were negative except for KRAS. However, because none of these markers have been demonstrated to be clear independent prognostic indicators, the information obtained from the mutation panel did not influence our decision to whether or not perform a prophylactic CND. As with any predictor of recurrence or mortality, interventional studies are required to determine which at-risk patients may benefit from additional therapies, prior to the widespread utilization of these molecular studies in clinical practice to determine the extent of treatment.
Recently, however, two more markers have been identified and appear to confer an increased risk of tumor recurrence and tumor-related mortality: the TP53 and TERT mutations. These markers are currently being tested for in cytology specimens in an effort to provide more accurate tumor prognostication preoperatively. Nonetheless, TP53 mutations have been known to occur mostly in poorly differentiated and anaplastic thyroid cancers. More recent broad genotypic analyses identified TP53 mutations in 3.5 % of well-differentiated PTC and 11 % of well-differentiated follicular thyroid carcinomas [61]. PTCs in this series that were positive for TP53 mutation also showed mutation in BRAF and developed lung metastases. All of the TP53-positive follicular thyroid carcinomas showed no other coexisting mutations and were oncocytic, while 75 % were widely invasive.
TERT mutations are found in 7–22 % of PTC and 14–17 % of FTC. There is a significantly higher prevalence in dedifferentiated thyroid cancer [62–65], in addition to PTC carrying the BRAF mutation [62, 63]. In the largest reported series by Melo et al. [62], TERT mutation was found to be an independent predictor of disease-free survival and mortality for DTC. Furthermore, the combination of a TERT mutation and a BRAF mutation within the same tumor was associated with a high risk of structural disease recurrence [66]. These results suggest that these molecular markers may be helpful for risk stratification of thyroid cancer and will provide a significantly more accurate risk assessment than BRAF mutational status taken in isolation. However, the identification of a molecular marker that is predictive of aggressive behavior does not necessarily mean that more extensive surgery, such as a prophylactic CND, or other aggressive therapy (e.g., radioiodine remnant ablation) will have a significant impact on clinical outcomes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree