2 The Conventional Treatment of Cancer: The Case of Non-Small Cell Lung Cancer
2 The Conventional Treatment of Cancer: The Case of Non-Small Cell Lung Cancer
 Introduction
Introduction
The purpose of this chapter is to review critically the three main conventional treatments for cancer: surgery, radiotherapy, and chemotherapy. I will illustrate problems involved in assessing their effectiveness by focusing on one kind of malignancy, non-small cell lung cancer (NSCLC). I have chosen lung cancer because it is among the most prevalent causes of cancer death worldwide and NSCLC is overwhelmingly the most common type of cancer at this anatomical site. I shall discuss the effectiveness of the standard methods in the treatment of various stages and substages of the disease.
The central question is whether conventional treatments, singly or in combination, have been proved to be effective and reasonably safe. There are many definitions of effectiveness, which leads to widespread confusion. Some people think effectiveness should be measured by the complete removal en bloc of a growth and its surrounding tissue; by the prevention of local, regional, or distant recurrences; or by the shrinkage of measurable tumor for a prescribed length of time.
Ideally, a description of effectiveness involves a comparison with patients who do not receive the treatment in question but receive a placebo or “sham” treatment. The effectiveness of any treatment must also be studied in the context of its long-term impact on the patient’s quality of life (QOL).
The best way of attaining such evidence is through prospective, randomized controlled trials (RCTs). According to the Institute of Medicine (IOM) of the US Academy of Sciences, an RCT is:
“… a formal study carried out according to a prospectively defined protocol. It is intended to discover or verify the safety and effectiveness in human beings of interventions to promote well-being, or to prevent, diagnose, or treat illness … Properly conducted clinical trials are a necessity in health care because very few interventions produce such large or striking results that they can be evaluated by observation alone” (1).
What Are the Characteristics of a Successful Clinical Trial?
According to the IOM:
“To generate the most reliable information, clinical trials require certain design characteristics (particularly assignment of participants to interventions by ‘randomization’) and they must include enough participants to exclude the play of chance as a likely explanation for results. … Regardless of the sophistication and complexity of the design and analysis, the question of whether ‘a’ is better than ‘b’ is the essence of the clinical trial” (1).
Since the end of World War II, clinical trials have been widely used throughout the industrialized world for the testing of new drugs and vaccines. After the well-known thalidomide disaster, and the consequent enhancement of the powers of the US Food and Drug Administration (FDA) in 1962, the US government has required clinical trials before it will allow any new diagnostic procedures or therapeutic agents onto the marketplace. (As I shall explain below, this standard has been eroded in recent years.)
RCTs should be large enough to yield a meaningful result (be adequately powered) and should hopefully involve more than one medical center. The RCT is the “gold standard” of testing. There is a perception among both medical professionals and the lay public that the conventional treatments for cancer (primarily surgery, radiation, and chemotherapy), which are in use worldwide, have been proved through this rigorous scientific process. In fact, the presence or absence of RCTs is supposed to form the boundary line between conventionally proved treatments and those nonconventional treatments that are collectively referred to as conventional and alternative medicine (CAM). Any claims of effectiveness for CAM is routinely met with the rejoinder: “Where are the RCTs to support such approaches?”
We immediately confront a problem: the focus of FDA reform in the 1960s was pharmacological agents. But two of these three main anticancer modalities, surgery and radiation, were excluded from the stringent 1962 FDA regulations. In legal parlance, they were grandfathered into US law (i. e. accepted based solely on their prior widescale use). Essentially, no formal approval process was deemed necessary before surgery or radiation therapy was employed as treatments for cancer patients. This exemption covered not just past treatments but future ones as well: cancer surgeons and radiation oncologists were essentially given carte blanche from the US government to introduce new or modified techniques for treating cancer. Other governments followed suit.
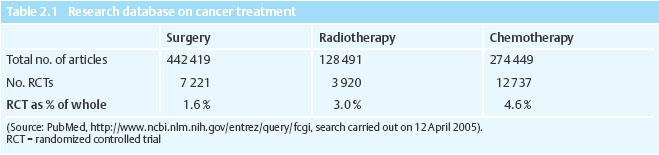
Perhaps for that reason, clinical trials of these two older modalities are more limited in number and scope than they are for drug-based therapies. In fact, on a percentage basis, there are approximately three times as many RCTs of cancer chemotherapy as there are for cancer surgery (Table 2.1).
However, despite this legal carte blanche, the scientific requirements of proof obviously remain the same for surgery or radiation as for any other treatment. No treatment can be considered effective until and unless it has been subjected to the highest possible standards of proof. Whenever feasible and ethical, this is the RCT. A World Health Organization publication addresses this question. “Radiotherapy must justify its place in the armamentarium of cancer-fighting technologies. Not only must it constantly be subject to clinical trials evaluating its role, it must also be reviewed in terms of its cost–benefit and utility in the circumstances in which it is used” (61). The same should be said of cancer surgery and chemotherapy.
 History of Clinical Trials
History of Clinical Trials
The idea of comparing one regimen or treatment directly with another is very old (5, 15). There is even reference to an early “clinical trial” in the Old Testament. Daniel and his companions were resolved to eat and drink a purely vegetarian diet. But Babylon’s chief palace official feared that “if you eat something else and end up looking worse than the other young men” the King might be angry. And so Daniel proposed the following test: “For the next ten days,” he said, “let us have only vegetables and water at mealtime. When the ten days are up, compare how we look with the other young men, and decide what to do with us…. Ten days later, Daniel and his friends looked healthier and better than the young men who had been served food from the royal palace” (12).
The foundations of the modern RCT were laid in the nineteenth century. A great French physician, Pierre Charles Alexandre Louis (1787–1872), wrote that “the edifice of medicine reposes entirely upon facts, and that truth cannot be elicited, but from those that have been well and completely observed” (45). Through a statistically driven study, Louis showed that bloodletting (the cornerstone of the conventional medicine of his day) was much less effective for inflammations than had previously been supposed. Louis declared that the rate of death of different patient populations “can only be attributed to the employment, or omission, of bloodletting” (44).
“In clinical medicine today there is a growing demand for adequate proof of the efficacy of this or that form of treatment. Often proof can come only by means of a collection of records of clinical trials devised on such a scale and in such a form that statistically reliable conclusions can be drawn from them. However great may be our aversion to figures, we cannot escape the conclusion that the solution of most of the problems of clinical or preventive medicine must ultimately depend on them” (30).
After World War II “it was no longer possible for the clinician, however distinguished, to discuss the prognosis and treatment of disease unless his words were supported by figures,” wrote L.J. Witts, Nuffield Professor of Clinical Medicine at Oxford University. “The accelerating pace of discovery entailed the constant trial of new remedies, whose value could not be left to be determined by the slow processes of time and fashion as in the past” (85).
Hill’s first clinical trial, completed in 1946:
• defined in advance the characteristics of who would, and would not, be treated
• objectively documented the response to treatments
• established a neutral committee to define the ethical issues posed by the trial.
One commentator noted at the time: “A notable feature of this trial was the frank realization by all concerned of the fallibility of human judgment in general and of clinical and radiological judgment in particular. … The principle of the elimination of personal bias is fundamental in all experiment, but it is of particular importance in clinical research.
Thus, in the selection of patients for inclusion in either treated or control groups, the final decision was made purely on a chance basis” (63).
In the last few decades, RCTs have emerged as the universally acknowledged gold standard of medical research (48). Almost a quarter of a million controlled trials have been carried out around the world (9). There are presently at least 2200 ongoing clinical trials available to cancer patients, publicized through US government websites such as www.cancer.gov and www.clinicaltrials.gov.
 Surgery as a Cancer Therapy
Surgery as a Cancer Therapy
Surgery has been called “the mainstay of therapy for malignant neoplasia” and “the most effective curative and palliative treatment for most solid tumors” (47). Its first uses actually predate recorded history. Yet it is only within the last century or so that surgery has become a respected profession. According to a standard textbook, “Throughout most of recorded history, surgeons have been the objects of societal opprobrium because of the unspeakable perioperative suffering endured by their patients” (49).
Before the late nineteenth century, extensive surgery more frequently resulted in death than in the cure of the patient. It was the discovery of anesthesia that led to a dramatic improvement in cancer surgery and its growing acceptance. For example, in the decade before the introduction of anesthesia the Massachusetts General Hospital in Boston performed just 400 operations. But from 1894 to 1904, over 24 000 operations were performed there.
The greatest innovations in cancer surgery were associated with Central European doctors, such as Theodor Billroth (1829–1894) and his student Emil Theodor Kocher (1841–1917). In America, William Stewart Halsted (1852–1922) transplanted the German model of organized postgraduate surgical education to America. In 1890, he described the radical mastectomy that bears his name. This Halsted operation was so extensive that it undeniably caused a great deal of suffering for many of the patients who survived. But, presumably, it was dramatically more effective at saving or prolonging patients’ lives. This operation became the prototype for radical cancer surgery at every anatomical site.
The use of the “Halsted radical” was based on a theory that was itself not subjected to experimental testing. This theory was that cancer spread in a stepwise fashion through the tissues and then through the lymphatic system, and that essentially cancer was a local or regional, not a systemic, disease. Halsted said that breast cancer spread through first and second order regional lymph nodes. In a famous lecture, he spoke of how breast cancer “in spreading centrifugally, preserves in the main continuity with the original growth, and before involving the viscera may become widely diffused along surface planes” (49).
This theory turned out to be unsupportable. Yet enormous numbers of patients were subjected to drastic operations without adequate experiments to test this underlying hypothesis. Since breast cancer was considered a “local affection,” surgeons were exhorted to apply “greater endeavor with the cognition that the metastases to bone, to pleura, to liver are probably parts of the whole …” (27). Here was the rationale for radical resection en bloc of the fascia of the major muscles, and for wide resection of the skin of the breast … and eventually to the “extended radical mastectomy,” in which excision en bloc was extended to include the internal mammary and supraclavicular lymph nodes and even portions of the bony thorax.
Halsted supported his theory with statistics on the survival of his own patients. In a retrospective case series, he reported five-year “cures” in 75 % of the 60 patients whose cancer had been confined to the breast, 31 % of the 110 patients who had had axillary metastases, and 10 % of the 40 patients who had both axillary and supraclavicular lymph node involvement. The operative mortality was just 1.7 %, an astonishingly low figure for the time.
The numbers certainly appear impressive. Clearly, Halsted was a skillful operator and a careful reporter. But no case series provides a synchronous control group with which its outcome can be compared. Halsted’s results appeared better than those of his contemporaries. But better results could be due to a number of biases inherent in the manner in which the patients were selected or the data interpreted. On what scientific basis did Halsted claim that his patients’ outcomes were better than what would have been achieved with other (e. g., less radical) approaches?
In arguing for the superiority of radical surgery, there was an unspoken assumption that meaningful comparisons were made to other practices (published or unpublished case series), past hospital records, or other historical data. This, as it turns out, was not a reasonable assumption. Biostatisticians today routinely expound the many biases inherent in case series or historical comparisons (52).
In fact, even at the time there was a growing awareness of the statistical deficiency of Halsted’s argument. Major Greenwood complained that Halsted failed to consider age distribution when he reported statistics on the success of his operation. In a letter, he observed that “surgeons … are mostly at the intellectual level of plumbers, in fact just well-paid craftsmen. I should like to shame them out of the comic opera performances which they suppose are statistics of operations … a really decent set of figures from such a panjandrum as Halsted would go a long way” (44).
It would be decades before the criticism of bio–statisticians carried any weight with the surgical profession. In the interim, Halsted’s radical approach spread from Johns Hopkins Hospital around the world:
“Halsted’s intellectual approach to the surgical management of cancer was widely adopted and eventually applied in one form or another to most solid tumors” (47).
The radical operation was primarily an American innovation. “The attention given scientific advances was especially great in a culture that had always placed a high value on tangible results. Physicians, it seemed, were at last exhibiting the ‘can do’ dexterity that was the essence of American ingenuity,” wrote one historian of cancer (58).
This “can do” attitude soon routed the “therapeutic nihilism” that had characterized much European thinking about cancer in the nineteenth century. For example, surgical resection of head-and-neck cancers became more common, especially in the 1940s and 1950s under the leadership of Dr. Hayes Martin of New York and his students. In 1906, Dr. George Crile, Sr. of Cleveland pioneered the radical neck dissection. By the 1950s, this was performed en bloc with the resection of the tumor itself.
Dr. Chevalier Jackson (1865–1958) of Philadelphia refined the techniques of tracheostomy. The first excisions of the abdominal colon took place in the decade between 1894 and 1903. This involved creation of a colostomy, followed by staged tumor excision and bowel closure. In 1908, W. Ernest Miles of London developed abdominoperineal resection for rectal cancer. Until recently, this remained the “treatment of choice” for this disease. “This operation was truly radical,” according to two surgeons, “committing all patients to a permanent end colostomy and at least half of the male patients to sexual disability” (47).
Halsted himself and then Dr. Allen O. Whipple (1881–1963) of New York carried out a radical operation for pancreatic cancer called pancreaticoduodenectomy (83). This “Whipple procedure” is still in use. But it was Dr. Alexander Brunschweig (1901–1969), also of New York, who took radical surgery to its ultimate extreme with the hemicorporectomy (translumbar amputation). This operation, which involved removal of half of the human body, was attempted in a few unfortunate patients. As one textbook comments:
“This practice was a logical, albeit extreme, extension of the doctrine that surgical resection of cancer can result in cure provided a satisfactory margin of normal, uninvolved tissue is taken beyond the limits of tumor spread” (47).
None of these treatments was established because of randomized controlled trials, or anything approaching what we would today consider proof of effectiveness. They were based on a widely held theory that cancer was a local disease and that the more extensive the surgery that was attempted, the better would be the survival results. This became the prevailing dogma of cancer surgery for decades.
But where was the experimental justification by which so many people were cut open, or even cut in half, in the name of radical surgery? There was an almost complete lack of controlled studies. Naturally, we can hardly demand randomized trials from an era in which they were all but unknown. However, after World War Two, and especially after 1962, RCTs became routine in the pharmacological field, yet were still almost unknown in the arena of cancer surgery.
The ultimate in untested, unscientific practice involved the treatment of malignant melanoma. “For much of this century,” Moffat and Ketchum of the University of Miami School of Medicine wrote in 1994, “the surgical management of primary cutaneous malignant melanoma was based on a detailed clinicopathological description of a single case by William Sampson Handley in 1907 … Surprisingly, with no other supporting evidence, Handley’s principles became established in surgical teaching and practice and defined the surgical standard of care for melanoma for many decades” (47, 49).
These firmly held ideas hardened into dogma. Richard Evans, MD, a cancer surgeon, has recalled his own medical education in the 1960s and 1970s:
“Surgeons seemed to work by a rigid set of rules meant to be heeded, but seldom questioned. Cancer surgeons relied upon ideas that had been passed down for almost a century. The number one rule of cancer surgery was to remove every single cancer cell, and every cell that may become cancerous. This was called radical surgery. Radical surgery was considered necessary in order to control the disease and ultimately cure the patient. Though this sounded reasonable, its functional and cosmetic consequences were sometimes devastating” (17).
A brake was finally put on radical surgery by a combination of advances in scientific understanding (derived in part from RCTs) and a widespread rejection on the part of patients and the general public of what were seen as surgeons’ excesses. The public’s mood was already summarized in 1906 by playwright George Bernard Shaw in the Preface to Doctor’s Dilemma:
“The test to which all methods of treatment are finally brought is whether they are lucrative to doctors or not. … Nobody supposes that doctors are less virtuous than judges; but a judge whose salary and reputation depended on whether the verdict was for plaintiff or defendant, prosecutor or prisoner, would be as little trusted as a general in the pay of the enemy. To offer me a doctor as my judge, and then weight his decision with a bribe of a large sum of money and a virtual guarantee that if he makes a mistake it can never be proved against him, is to go wildly beyond the ascertained strain which human nature can bear.”
Shaw also noted the lack of scientific proof for the radical surgery of his day: “The large range of operations that consist of amputating limbs and extirpating organs admits of no direct verification of their necessity.”
The public grew increasingly concerned about the functional and social consequences of cancer operations (47). Some people, faced with radical operations, refused treatment. In fact, despite numerous and vociferous assertions to the contrary, increasing the magnitude of operations had not been scientifically demonstrated to result in a proportionate increase in “cures” or improved survival rates for solid tumors.
The 1970s and 1980s were marked by the ascendance of a more humane philosophy of more limited or “conservative” surgery. This type of surgery attempted to preserve body form, function, and parts. Surgeons, commented one textbook, “were initially slow to grasp the need for less radical surgery … (49).” Eventually, conservative surgery became standard treatment for a number of malignancies, most prominently breast cancer.
Critics of radical surgery resorted to RCTs to prove that alternative and competing methods were equally, if not more, effective than radical operations. As Major Greenwood anticipated, the clinical trial became a weapon in the fight to reign in unwarranted claims of success and to make surgery a more scientifically based discipline. For many kinds of cancer, the conservative approach (sometimes supplemented by radiation and chemotherapy) won out. But the overall effectiveness of surgery still rests more on common sense than it does on randomized clinical trials.
 Radiation as a Cancer Therapy
Radiation as a Cancer Therapy
Roentgen rays were discovered at the end of 1895 by Prof. Wilhelm C. Roentgen of the University of Würzburg, Germany. He was rewarded with the first Nobel Prize in Physics for this epochal discovery. By 1896, early investigators (including Thomas Alva Edison in America) found that these mysterious rays could not only visualize the interior of solid objects, such as the human body, but could have a destructive effect on living tissues.
The first attempts to use radiation as a cancer treatment were conducted in January 1896 by a homeopathic medical student in Chicago, Emil H. Grubbß. In Europe, better equipped laboratories were not far behind. On 3 February 1896, J. Voigt of Hamburg treated a case of carcinoma of the nasopharynx and reported for the first time pain relief through radiation therapy (79). In France, V. Despeignes applied roentgen rays to two patients, one of whom had cancer of the mouth, the other of the stomach. In the first case, severe pain ceased almost immediately. In two patients with inoperable breast cancer “daily irradiation soon relieved the excruciating neuralgic pain … (22).” It is generally accepted that Dr. Leopold Freund of Vienna (21) was “the first to use roentgen rays logically and scientifically within the limits of the age” (53).
Two facts stand out from the early experiments with roentgen rays and later with radium (discovered by the Curies in 1902): they could often relieve pain and palliate other symptoms of cancer; and they could sometimes shrink or even destroy established tumors. Thus, it is not surprising that radiation therapy grew in popularity and that enormous hopes were invested in this new form of treatment. For the first time, an alternative to surgery seemed possible and the mood of pessimism that characterized nineteenth-century thinking about cancer began to dissipate.
The reputation of radiation therapy rested, and continues to rest, on these two obvious facts. However, pain relief and tumor destruction does not tell the whole story of radiation treatment. For radiation, as was soon discovered, was a two-edged sword, with many harmful effects on the body.
It was soon noted that roentgen rays could burn the skin or irreparably damage internal organs or structures. In 1896, Dr. D.W. Gage of McCook, New Brunswick, writing in New York’s Medical Record, noted cases of hair loss, reddened skin, skin sloughing, and strange growths or lesions. “I wish to suggest,” he wrote mildly, “that more be understood regarding the action of the roentgen rays before the general practitioner adopts them in his daily work” (81).
Roentgen rays also turned out to not just “cure” cancer but to cause it as well. In fact, hundreds of radiologists and technicians died horribly, many of them of cancer, as a result of underestimating the dark side of their new technique. It was also found that tumors obliterated by radiation often recurred, sometimes in distant organs, and that these recurrences were difficult to treat with any conventional technique. After the explosion of the atomic bombs at Hiroshima and Nagasaki, detailed studies over decades showed that those exposed to radiation suffered many long-term harmful effects. In making an evaluation of the positive or negative effects of radiation, therefore, one simply cannot make a judgment based on its immediate impact on tumors. One needs to study the effects of the exposure over a period of time, preferably for decades.
Such questions can best be answered by evaluations conducted in the course of randomized controlled trials. But RCTs are difficult and expensive to conduct. Since they are not legally required for radiation by the FDA, they are often lacking. The vast majority of evaluations of the effectiveness of radiation therapy are based on reports of case series, not RCTs. When RCTs have been performed, they sometimes show that radiation is ineffective in prolonging overall survival of the participants. Thus, as a general rule, the safety and effectiveness of either adjuvant or therapeutic radiation therapy has not been adequately demonstrated through rigorous trials.
 Chemotherapy as a Cancer Therapy
Chemotherapy as a Cancer Therapy
Chemotherapy is more scientifically based than either radiotherapy or cancer surgery. In the five years between 1999 and 2004, over 75 000 peerreviewed articles have appeared in Medline (PubMed) on the topic of cancer chemotherapy. An additional tens of thousands of abstracts have been presented at the American Society of Clinical Oncology (ASCO) and the American Association for Cancer Research annual meetings. Out of this vast amount of research, over 115 new approvals have been made by the US Food and Drug Administration (FDA) for drugs used in the treatment of cancer patients (see http://www.fda.gov/cder/cancer/druglistframe.htm). All of these are based on clinical trials of some sort. But what is the nature and quality of these trials? Has the current generation of anticancer drugs been proved to improve the overall survival of cancer patients?
In fact, randomized controlled trials (RCTs or phase III studies) are inconsistently performed in conventional oncology before or after the approval of a new drug. It is very rare for a trial to compare a drug with placebo (sham treatment) or even best supportive care (BSC). When studies are reported as positive that is usually in comparisons with other agents, or it is a measurement of improvement in surrogate markers rather than of increased overall survival.
Drugs may be reported to increase survival, but upon closer examination this sometimes turns out to be an increase in “relapse-free survival,” or “time to recurrence,” and not an actual increase in median overall survival. The latter are important figures, to be sure, but the most important and reliable figure is that of overall survival. Otherwise, the patient may experience an increase in the relapse-free period, but no increase in his or her actual life expectancy. Such a patient may only be benefited in a psychological sense. Yet real improvements in overall survival for the solid tumors of adults are rarely demonstrated in rigorous trials with chemotherapy.
Surveys have shown that the two outcomes that cancer patients seek from chemotherapy are: first, an improvement in quality of life; and second, an increase in their actual survival. Tumor shrinkages in and of themselves are not a high priority (6). The “Grand Illusion of Chemotherapy” is the idea that the shrinkage of tumors, improvement in tumor markers, or increased relapse-free survival, necessarily correlates with actual benefit to patients.
But developmental oncologists tend to concentrate on the shrinkage of tumors or perhaps relapse-free survival. “Responses” are often defined in terms of tumor shrinkage, and drugs that shrink tumors are called “active agents.” The FDA defines a complete response as a complete disappearance of all clinical and radiographic signs of cancer for one month or more. A partial response refers to a 50 % or greater decrease in measurable tumor size for one month or more. In the past, the FDA also required proof of life prolongation. But this stringent requirement led to very few drug approvals. From the 1940s to the mid-1990s, in fact, only about three dozen anticancer drugs were approved by the FDA, less than one per year. The FDA’s reluctance to approve drugs based on shrinkages angered the pharmaceutical industry, as well as some oncologists and patient activists. So, in the mid-1990s the government relaxed these requirements, and since then FDA has approved many new drugs.
Finally, there is a huge economic dimension to the pharmaceutical treatment of cancer. Treating cancer with drugs has become big business. The following news item from the New York Times Website (12 October 2000) speaks for itself:
“Genentech, Inc. on Wednesday reported a 27 % gain in third-quarter profits … driven by sales of cancer drugs, Herceptin and Rituxan. Third-quarter sales of Herceptin increased 52 %, to $ 72.6 million … Sales of Rituxan increased 62%, to $ 117.9 million.”
In other words, just this one pharmaceutical company is earning about US$ 750 000 000 per year from the sale of two of its anticancer agents. In the first years of the twenty-first century, it became common to hear talk of billion dollar sellers in the cancer drug field. By 2006, global spending on cancer drugs is predicted to total $ 31.7 billion, up from $ 22.3 billion in 2004, according to the consulting firm of Bain & Co. That makes cancer the fastest growing drug category (89). Bristol Myers should therefore quickly recoup what seemed like a ridiculous research investment of US$ 2 billion in a very short time, thanks to the FDA’s compliant policy of accelerated approval. Oncologists who sell anticancer drugs in their offices–the so-called “chemotherapy concession”—will also benefit from this largesse (64).
 The Conventional Treatment of Non-Small Cell Lung Cancer
The Conventional Treatment of Non-Small Cell Lung Cancer
I would now like to examine the actual record of these three approaches in the treatment of one particular kind of cancer. Lung cancer is one of the most prevalent causes of cancer death worldwide. In the United States, there were an estimated 186 550 new cases of cancer of the respiratory system in 2004 (4). By another estimate, the world market for cancer therapeutics passed US$ 42 billion in 2004 and is expected to post rapid annual growth in the next five years, according to a study by Kalorama Information, New York, NY. New biologics and adjunctive therapy products are said to be the main growth drivers (68a).
Annually, cancers of the lung and bronchus accounted for 91 930 US deaths among men and 68 510 among women (2004 figures). It is three times as prevalent a cause of death among men as its nearest contender, prostate cancer (with 29 900 deaths) and has long since overtaken breast cancer as the leading cause of cancer death among women as well (68 510 vs. 40 580). In Germany, in recent years it has accounted for approximately 40 000 deaths annually (28 675 in men and 9296 in women) (84).
There are numerous books and thousands of scientific articles on the treatment of primary non-small cell lung cancer (NSCLC). However, most discussions are descriptive in nature. They accept and describe the therapeutic practices that are generally performed. Few of them examine the question of therapeutics in a critical way, with an eye on the quality of the evidence that is put forward to justify the claims of various treatments.
One exception to this rule is the Physicians’ Data Query (PDQ) system of the National Cancer Institute (NCI). This US government database attempts to assess the quality and rigor of studies on which treatment decisions are made. The PDQ statements attempt to introduce a more scientifically based evaluation of the evidence. These statements also represent the official position of the US government on the treatment of cancer in its various stages. It is arguably the most influential body of literature on cancer treatment on the Internet. For these reasons, I will most often cite its statements in discussing the conventional treatments of this disease. (Note: in 1999, the author was appointed an advisor to the PDQ Adult Treatment Editorial Board.)
The PDQ editorial board sometimes uses a formal ranking system to help the reader judge the strength of evidence linked to the reported results of a therapeutic strategy. (Unfortunately, the application of this system remains sporadic.) We shall therefore follow its presentation on the effectiveness of the various conventional treatments, with a focus on the results attained through RCTs.
One problem we immediately encounter is the existence of different staging systems for this disease. We have followed the PDQ nomenclature of four stages, with stage III divided into two substages (IIIA and IIIB). The PDQ also recognizes and discusses a “stage 0” disease. However, each stage can also be characterized according to the TNM system as well (which classifies a tumor by its size, its regional lymph node involvement and distant meta-static spread). In addition, the American Joint Committee on Cancer (AJCC) and the Union Internationale Contre le Cancer (UICC) have proposed changes that will essentially divide stages I-III into two substages apiece. This will be more accurate but will make historical comparisons even more problematical.
The Surgical Treatment of Non-Small Cell Lung Cancer
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree