Fig. 33.1
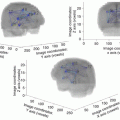
Axial FLAIR-weighted MRI showing a typical illustration of a “false DLGG”, which is in fact a highly diffuse glioblastoma. In its initial phase of low density (left), such a glioblastoma really looks like DLGG, as there is a low cell density and no contrast enhanced area. However, due to an unexpected size increase on a second MRI performed 6 weeks after the first one (left), corresponding to a high growth rate (velocity of diametric expansion >8 mm/year), the diagnosis of “false DLGG” can be made before to obtain histo-molecular results. In this case, because a (supra)complete resection is not feasible due to an invasion of the subcortical connectivity, resection will leave behind a high proportion of tumors cells, with a high proliferation rate. Hence, only treatments targeting tissues beyond the visible tumor margins—hence a combination of chemotherapy and radiation therapy—could potentially stabilize this tumor. As a consequence, the onco-functional balance is low in this example
33.2.1.3 Specific Situations of Potentially High Oncological Benefit
It is now well recognized that DLGG might exhibit spatial heterogeneities [15]. New imaging techniques enable to detect such heterogeneities. In particular, dynamic 18F-FET-PET allows to identify foci of decreasing time-activity curves, that correlate with histological grade [16]. Similarly, the role of perfusion imaging to detect such hot spots is still investigated. Therefore, although this has not be proven, it can be anticipated that removal of such malignant foci within an otherwise DLGG could improve oncological benefit of surgery, even though the entire glioma was not completely resected.
Finally, epigenomic and genomic landscapes and their evolution in the course of glioma growth are being progressively deciphered [17–20]. Such studies evidenced a branching pattern rather than clonal evolution. Importantly, the different branches are located in different spatial spots in the tumor [19, 20]. Thus, it can be anticipated that a better modeling of this genomic evolution could allow to identify a hot spot within the tumor bearing a branching clone of higher aggressiveness. Surgical removal of this specific area could result in enhanced oncological benefit, even if the resection is partial.
33.2.1.4 Factors Influencing Functional Risk
It has been shown that awake surgery with intraoperative neurological and neuropsychological testing and monitoring greatly reduces the functional risk of DLGG surgery [21]. Nevertheless, it should not be forgotten that immediate postoperative cognitive evaluations report a deterioration in most of patients, with a (quite) complete recovery after 3 months of intensive rehabilitation. It is acknowledged that this full cognitive recovery is made possible by the plasticity of distributed networks [8]. But the sine qua non condition for network normalization by postoperative plasticity is the surgical preservation of a minimal core of long-range connectivity [22–24], including but not limited to superior, middle and inferior longitudinal systems, inferior fronto-occipital fasciculus, cingulate fasciculus, frontal aslant tract, and callosal fibers. Such axonal pathways are essential to maintain the small-world topology of brain networks [25]. Therefore, these functional white matter tracts should be imperatively identified by direct subcortical electrostimulation mapping in all DLGG patients, whatever the tumor location, and they should serve as limits of surgical resection [26].
Considering that the neuronal areas per se of this core of connectivity are safely controlled thanks to intraoperative monitoring, small deep arterial infarcts remains the major source of damage of the long-range connectivity. Statistical map of diffusion hypersignal with decreased ADC (an MRI signature of acute ischemia) after glioma resection is lacking, as well as the functional correlates of such small strokes depending on their topography. It can be hypothesized that any stroke encompassing the minimal common brain [23] (i.e. the basic core of associative connectivity), would impact high level cognitive functions.
Moreover, for a similar insult of the connectome, the degree of recovery will vary from one patient to another, depending on their level of plasticity reserve. Hence, estimating the functional risk of a given patient means evaluating its plasticity reserve, which is currently not straightforward. First of all, a slight deterioration in some of the preoperative cognitive scores reflects that neuroplasticity has already spent much of its reserve. Interestingly, preoperative infiltration by glial cells of the connectivity of the ventral stream has been shown to correlate with a decrease of semantic fluency scores [27]. In other words, preoperative infiltration by the glioma of the minimal core of connectivity can hamper the reshaping processes of networks reconfiguration, precluding a fully effective functioning. This preoperative infiltration of the connectome has also to be taken into account regarding postoperative plasticity. Let us consider for example the effect of ILF resection on lexical access [28]: the degree of recovery could differ depending on the degree of preoperative infiltration of the other associative tracts involved in this function (inferior fronto-occipital fasciculus, long direct segment and short posterior segment of arcuate fasciculus [22]). This would be especially true in a fast growing glioma, because the axonal dysfunction induced by glioma infiltration has an impact on the process of neuronal synaptic reweighting underlying network normalization. In the same vein, postoperative radiation therapy on a residue left in the connectome could seriously compromise the chances of recovery [29].
Finally, age is a major factor of plasticity potential: as age increases, plasticity decreases. Surprisingly, there are no study in the literature reporting the influence of age on cognitive recovery after awake surgery of DLGG. In our experience, we observed that after 55 years of age, recovery took longer (between 8 and 18 months) in comparison to younger patients (for example, median time to work resumption of 4 months in [30]).
33.2.1.5 The Definition of Functional Risk: A Personalized Assessment
Since postoperative cognitive evaluations are performed on a routine basis, our knowledge of the impact of surgery on high-level functions has greatly improved, leading to a better preoperative personalized estimation of functional risk [31]. To this end, the importance of the preoperative patient interview cannot be overemphasized. Patient should clearly formulate his wishes regarding which kind of functions should be primarily preserved. This will depend on its way of life, including profession, hobbies, and social interactions [32]. For example, it has been recently recognized that proper names retrieval is frequently impaired after left dominant temporal lobectomy [33, 34]. In any profession requiring a high level of proper names retrieval (for example when you need to deal with a lot of client names), such deficit precludes work resumption—while for some other patients, such a deficit would not affect quality of life. Another example is provided by fine motor functions : it has been reported that resection of SMA glioma without identifying networks of motor control [35–37] results in long lasting trouble in bimanual coordination [30, 38]. Again, such deficit goes unnoticed for some patients, while it would dramatically impact the professional life of a musician or a tennis player. In a similar way, the ecological consequences of the resection of optic radiations (causing an hemianopia) is not of the same importance whether the patient absolutely needs to drive or not [7]. In the same state of mind, the level of cognitive control (i.e. executive functions) can be set preoperatively with the patient, and intraoperative task can be personalized accordingly. Several tasks requires a high cognitive load, including but not limited to, dual task, working memory tasks, or TMT part B. Preserving such high-level cognitive capabilities is probably more important for a manager than for a cleaning lady. The same principles apply to emotional functions, like mentalizing. The read the mind in the eye task allows to identify the low-level network of mentalizing [39], and it is anticipated that preserving this network is of utmost importance for patients with a profession requiring a high level of empathy, like medical doctor.
Finally, there is a controversy regarding the possibility to offer a complete resection that would come together with a high risk of neurological deficit [40–42]. Resection of tumors involving the anterior perforated substance (APS), usually insular or paralimbic gliomas [43], is a paradigmatic example of this ethical issue. Complete resection of the APS comes with a very high risk of hemiplegia. Surgeons agreeing with the moto “primum non nocere” would definitely not go beyond the functional responses elicited in the external capsule covering the APS, thus giving up to resect the tumor part within the APS. In this tumor location, some other surgeons plaid for informing patients about the two options (safe resection with suboptimal oncological benefit versus maximizing oncological benefit at the price of hemiplegia) and offer them to get involved in this decision, that is, to accept (or not) severe permanent deficit in order to increase the extent of resection. However, it is questionable whether the patient can objectively imagine how he could enjoy life with an hemiplegia and/or a marked cognitive deficit—because cutting the temporal stem to reach the APS will also induce definitive higher-order disturbances (in addition to the motor deficit related to the injury of the lenticulo-striate arteries when removing the APS itself), especially due to a damage of the inferior fronto-occipital fasciculus, even in the right hemisphere [44].
33.2.1.6 The Functional Benefit of a Surgical Resection
Functional improvement after resection of DLGG have been reported [30, 45, 46]. Among other explanations, this could be due to the relief of mass effect or to the better reorganization of brain functional networks after tumor removal [47]. Moreover, the positive impact of resection on epilepsy status should not be overlooked [48]. It has been suggested that resection of temporo-mesial structures could be offered for epilepsy control even if not infiltrated by the glioma [49]. This functional benefit (which might be of utmost importance given that seizure control is the key parameter allowing patients to keep their driving license) has to be weighted with the functional risk of verbal memory decline, which is a well known consequence of temporo-mesial structures removal.
Importantly, the functional risks of the surgery should also be weighted in comparison to the spontaneous evolution of the DLGG. Indeed, as reported above, in absence of any surgery, there is a progressive cognitive decline all along the natural course of the disease, related to the infiltration of the minimal common brain. Of note, in a old series, it was claimed that with an endpoint at 4 years, surgery was cognitively more risky than wait and watch management [50]. However, this result might be out of date, considering that surgery was not performed under awake mapping at that time. In addition, even assuming that this would be still valid nowadays (which is very unlikely taken into account the favorable neurological and neuropsychological outcomes when using intraoperative awake mapping [45, 46, 51–54]), a 4-years time-point is not enough to draw any conclusion, considering that median survivals have now reached more than 10 years. In fact, any surgery that would delay the infiltration of the connectome should help to delay cognitive decline. Hence, (supra-)complete resection while preserving the minimal core of connectivity provides a functional advantage compared to wait and see. On the contrary, if a residue is left within the infiltrated connectome, the functional advantage of surgery is only provided by the oncological advantage, that is by delaying malignant transformation. Indeed, it has been shown that the residue will grow at the same speed as before surgery [55], meaning that in this situation, the connectome infiltration will not be delayed by the surgery compared to wait and see.
33.2.2 Chemotherapy
33.2.2.1 Oncological Benefit
The oncological role of chemotherapy has been recently proven: median survival in patients treated with PCV concomitantly to radiation therapy was 13.3 years versus 7.8 in patients treated with radiation therapy alone [2]. Unfortunately, this study suffers so many flaws that its clinical utility is questionable. First of all, there is no information regarding the timing of the treatment. The abnormally high rate of contrast enhancement (between 60 and 65% in comparison to 15% in reference series of DLGG [3]) strongly suggests that many patients have been in fact initially monitored under a close wait and watch attitude, and included in the study only after onset of contrast enhancement. Moreover, it is not stated if this survival advantage is only observed in 1p19q codeleted patients. Indeed, the very same result was previously reported in grade III glioma [56, 57], with the additional result that the effect was only significant in patients with 1p19q co-deleted tumors. Nonetheless, as stated in [57], “Our data underscore that 1p/19q codeletion status is a marker, not a mechanism of sensitivity to PCV plus RT”, meaning that this parameter cannot be used to drive a therapeutic decision on an individual basis. Moreover, in any of these studies, the role of extent of resection was not appropriately accounted for. Subgroup analysis based on extent of resection objectively assessed on postoperative MRI would have been of paramount importance to individualize treatment decision according to this major prognosis factor [3, 6]. Last but not least, this trial comes without any relevant data regarding the functional status of patients. Indeed, in the long-survival arm (median survival = 13.3 years), we would expect a significant cognitive decline, subsequently to long-term adverse effect of irradiation. However, no cognitive assessment has been performed beyond 5 years of follow-up [2].
Otherwise, in the preliminary results of EORTC 22033 trial, even though it has been suggested that progression free survival is lower in patients with non-codeleted 1p19q tumors treated with upfront temozolomide rather than radiation therapy, no data has been reported regarding overall survival, preventing to draw any conclusion [58].
All in all, current knowledge supports the idea that PCV and temozolomide have a strong oncological benefit in 1p19q codeleted tumors (which is in accordance with previous studies reporting volumetric decrease under these regimens [59, 60]), and that there might be a synergistic effect with concomitant radiation therapy.
33.2.2.2 Functional Benefit
In terms of functional benefit, it is well known that chemotherapy can reduce dramatically the seizure frequency [61, 62]. Last but not least, it is obvious that temozolomide is so much better tolerated than PCV. It is striking that only 56% of patients could end the PCV protocol in the RTOG 9802 trial [2]. Hence temozolomide might be the best choice for optimizing the onco-functional balance at first line of chemotherapy.
Nevertheless, several questions remain unsolved: is there a loss of oncological benefit if a first line of chemotherapy is administered upfront, with the concomitant chemo-radiation kept for recurrence? If no, what is the optimal timing of first line of chemotherapy after the discovery of the DLGG: right after or delayed? Is there a difference in oncological benefit between PCV first line followed by temozolomide plus radiation at recurrence versus temozolomide first line followed by PCV plus radiation at recurrence?
33.2.3 Radiation Therapy
Current data on the real oncological benefit of radiation therapy are scarce. Indeed, it has been shown that in terms of survival, there is no benefit of early versus late radiation therapy in DLGG [63].
From a functional point of view, long-term adverse effects have been well described [64]. This study found a significant cognitive decline 7–10 years after irradiation, compared to a well matched population of DLGG patients without any irradiation. Currently, the factors that would allow to predict these late adverse effects in order to improve our evaluation of onco-functional benefit of radiation therapy are poorly known: are they related to age and cardiovascular parameters? to the volume of irradiation? to the location of the irradiated area, especially within or outside the minimial common brain—i.e. not only irradiation of the hippocampi but also irradiation of the functional white matter tracts? Interestingly, a recent series showed that diffusion tensor imaging can predict cognitive function deficit following partial brain radiotherapy for DLGG, in particular with an increased radial diffusion at the end of radiotherapy able to significantly predict decline in verbal fluency 18 months after irradiation [29].
Last but not least, short-term adverse effects are poorly described in the context of DLGG. Only a very recent paper reported a 20% rate of pseudoprogression [65], with a median delay of 12 months (range 3–78 months). In this study, no clinical symptoms were attributed to these pseudoprogressions, but one can fear that such reactions would be harmful whenever the irradiation targeted a residual tumor left by the surgeon for functional reasons.
These combined data about lack of survival benefit of early treatment and late onset of adverse effects grounded the recommendation to postpone radiation in DLGG whenever survival is expected to be greater than 7–10 years [66].
33.3 Second Level: Onco-Functional Balance of Treatments Integrated Within a Whole Sequence
Beyond the onco-functional balance intrinsically related to a given treatment, the choice of a treatment modality should be reevaluated in light of its interaction with associated treatments in the whole sequence. In this part, we propose to review some of these interactions.
33.3.1 Surgery as a Potentiating Neoadjuvant Treatment of Surgery
After a first surgery, brain networks reorganize, thanks to the plasticity, that is enhanced by intensive rehabilitation. Consequently, a delayed second surgery can be proposed, as functional limits have been pushed away from the boundaries of previous resection cavity, allowing to achieve greater extent of resection the second time while preserving brain functions [8, 67, 68]. Such a strategy can be envisioned only if plasticity mechanisms can take place. One condition is that the tumor growth is slow enough. In this perspective, the importance of assessing postoperative growth rates of residue cannot be overemphasized. The second condition is that the initial residue was left in a cortical epicenter outside of the deep core of connectivity. Such a cortical epicenter can be compensated after the first surgery thanks to network reshaping, while a residue within the connectome is much less likely to undergo such “functional silencing”.
33.3.2 Surgery as a Potentiating Neoadjuvant Treatment of Chemotherapy
To our knowledge, little is known about the benefit of reducing the tumor volume with the hope to increase the benefit/risk ratio of chemotherapy. However, one could speculate that, if chemotherapy can result in a stabilization of DLGG, the risk of malignant transformation will be significantly decreased if the tumoral volume under control by temozolomide or PCV is reduced by surgery first—especially when the postoperative residual volume is less than 10–15 cm3.
33.3.3 Chemotherapy as a Potentiating Neoadjuvant Treatment of Surgery
It has been proven that temozolomide can be proposed in a neoadjuvant setting [13, 69–71]. The goal is to reduce the tumor volume, up to the point that resection can be made at least subtotal (i.e. with a significant survival benefit). It is expected that this combination could be advantageous on a functional point of view: the response to chemotherapy should be correlated with a cognitive recovery, itself correlated to a normalization of functional connectivity. Even though this has not yet been demonstrated, there is nonetheless another argument in favor of this hypothesis. Computer modeling envision seizures as a signature of plasticity saturation [72]. Hence the reduction in seizure frequency under chemotherapy might be associated with a revival of plasticity, thus optimizing the surgical conditions. Accordingly, a study showed that cognitive status was very good in patients treated by neoadjuvant temozolomide, followed by surgical resection [70].
There is another rationale for this completion surgery. It has been suggested that temozolomide, by inducing DNA mutations, might contribute to the malignant transformation [73]. The surgical removal of a temo-induced aggressive clone can thus potentiate the chemotherapy.
Of note, this neoadjuvant chemotherapy can be applied at re-evolution of a residue left at first surgery, in particular if this residue is growing too fast (and thus impeding optimal remodeling of functional networks by plasticity): this could reopen the door to a second (or even third) surgical resection.
33.3.4 Chemotherapy as a Potentiating Adjuvant to Radiotherapy
As reported above, survival is better when chemotherapy follows immediately irradiation. There is a synergistic effect, that is not observed if the chemotherapy is administered later in time. Nevertheless, the trial by Buckner et al. did not address the question of starting first with chemotherapy alone, and delaying radiation therapy at a later stage [2]. Long-term results of the EORTC 22033 should provide some elements of response in the future.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree