Fig. 23.1
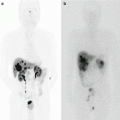
(a) Superior part of the pancreatic head. (b) Inferior part of the pancreatic head. (Arrow a) Soft tissue plane between antropyloric area of the stomach and the pancreas, (Arrow b) soft tissue plane between IVC and the pancreas, (Arrow c) plane between omentum and mesocolon and anterior inferior surface of the pancreatic head (D duodenum, G stomach, P pancreas, IVC inferior vena cava)
If the tumor is located anterior superior part of the pancreatic head, it would be safe not to try to separate prepyloric stomach from the pancreas head and just not to preserve the pylorus. If the tumor is located anterior inferior head, it would be safe not to separate omentum and mesocolon from the pancreatic head and remove them together (Fig. 23.2). Posteriorly located tumor can be exposed to posterior surface of the pancreatic head and invaded into loose tissue between the pancreas and IVC (Fig. 23.3). So, when the duodenum with pancreatic head is mobilized from retroperitoneum (Kocher maneuver), all the soft tissue between the pancreas and IVC should be completely removed so that IVC is clearly seen without covering any soft tissues.
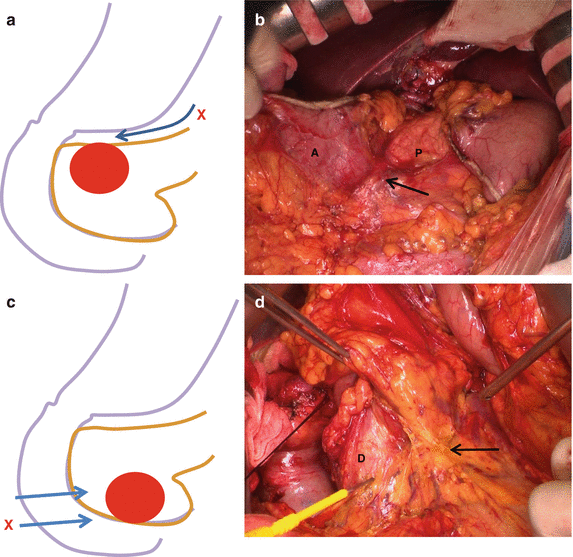
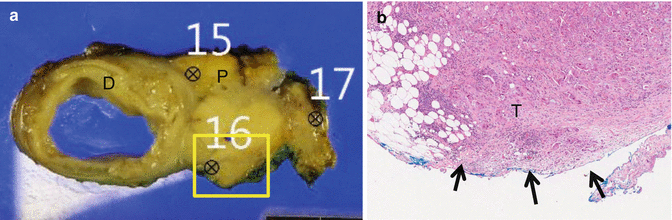

Fig. 23.2
(a) For tumors located anterior superior part of the head, it would be safer not to separate prepyloric stomach from pancreas head. (b) Arrow indicates the intact soft tissue between antropyloric area and pancreatic head. (c) For tumors located anterior inferior head, it would be safer not to separate covered omentum and mesocolon from the pancreatic head. (d) Arrow indicates pancreatic head covered with omentum and mesocolon (G gastric antrum, P pancreas, D duodenum)

Fig. 23.3
Potential tumor exposure of posterior surface. Posterior-located tumor as seen on the cross-sectional gross photo (a) can be exposed (arrow indicated) as seen in Fig. (b), that is, microphoto of yellow frame area of gross section (a). Pathologic report says “Pancreas posterior resection margin: presence of tumor, involved by carcinoma” (D duodenum, P pancreas, T tumor)
23.1.2 Level of Organ Transection
Organs to be resected are the bile duct, gastroduodenal segment, and pancreas. The level of bile duct cutting can be customized according to the tumor origin and location (Fig. 23.4). For pancreatic head cancer, common hepatic duct just proximal to the cystic duct insertion site is recommended. Common bile duct level transaction (distal to the cystic duct) is not recommended because sometimes cystic duct lumen and long redundant common duct make anastomosis complicated, even though its radicality is acceptable for pancreatic cancer. It would be oncologically safer to remove the whole extrahepatic common duct for common bile duct cancer.
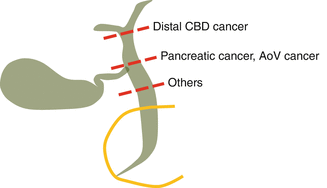

Fig. 23.4
Customized level of bile duct transection according to the tumor origin and proximal extent
There are some options for gastroduodenal transaction level (Fig. 23.5). In addition to conventional pancreatoduodenectomy and pylorus-preserving pancreatoduodenectomy (PPPD), pylorus-resecting or near total gastric-preserving pancreatoduodenectomy has been introduced recently as another option, mainly for preventing delayed gastric emptying after PPPD [2, 3]. If the pylorus is thought to have a significant physiologic function, it could be the surgeon’s choice whether they preserve the pylorus for preventing delayed gastric emptying. In author’s institution, PPPD is considered first unless there is any reason to do PD, and then the level of transaction can be customized between PD and PPPD according to various factors. For instance, PD is recommended for the tumor at anterior superior portion of the pancreas, and PPPD is recommended for tumors at inferior portion of the head of the pancreas (Fig. 23.6). If the duodenal perfusion condition is not good enough, other options can be tried, such as pylorus-transecting PD.
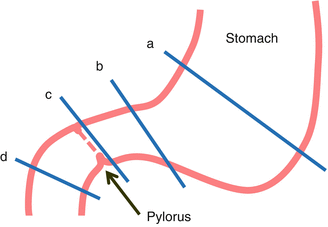
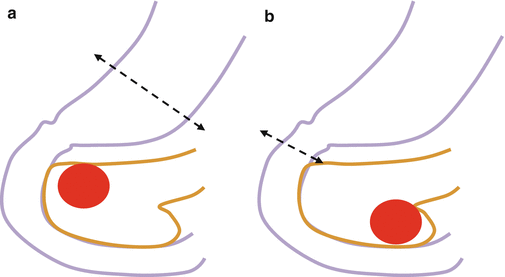

Fig. 23.5
Different options for gastroduodenal transection level between PD and PPPD ((a) classical pancreatoduodenectomy (PD), (b) subtotal gastric-preserving PD, (c) pylorus-resecting PD, (d) PPPD)

Fig. 23.6
Choice between PD and PPPD. PD is recommended for tumor at anterior superior portion of the pancreas (a) and PPPD is recommended for tumors at inferior portion-ventral pancreas (b)
Pancreatic neck transaction line should be customized according to the tumor location. A safe length of gross tumor-free segment has not been clearly defined. It would be difficult to define because of infiltrating nature of pancreatic cancer and associated inflammation of the pancreas, especially distal side of the tumor. In order to get free margin, 1 cm margin at least is recommended, so transaction line should be customized according to the tumor location.
Transection line can also be customized for technical reason. Pancreatic duct runs very close to the posterior surface of the pancreatic neck, so that anastomosis is sometimes very difficult. Transection at a little left side of the neck is recommended to get more centrally located duct for easier anastomosis (Fig. 23.7).
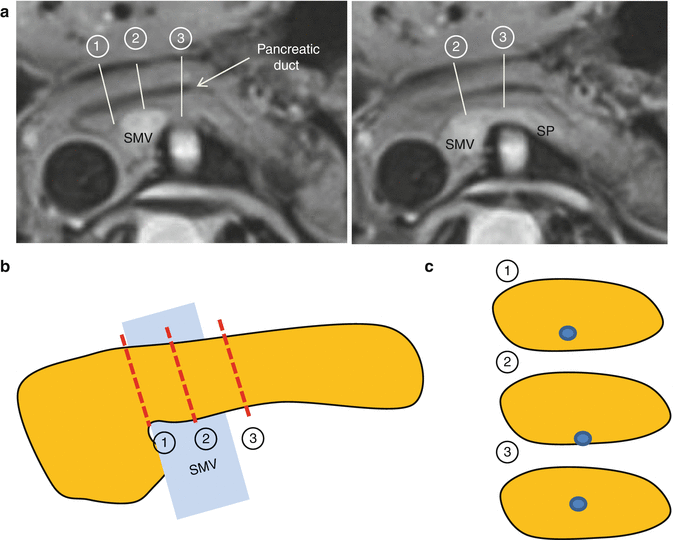

Fig. 23.7
Location of pancreatic duct at the cut section of the pancreatic neck (SMV superior mesenteric vein, SP splenic vein). (a) CT image and transection lines. Respective transection lines (b) and duct location on cut sections
23.1.3 Conventional Approach Versus “Artery-First” Approach
Separation of uncinate process from SMA as the first procedure (SMA-first approach) is what many surgeons stress nowadays [4]. Mesenteric approach is the most commonly adopted artery-first approach (Fig. 23.8). Most surgeons are used to conventional right side approach (SMA-last approach), but the mesenteric approach has advantages for the tumors involving uncinate process, especially in the cases of SMV/PV invasion or suspicious SMA invasion. It is considered to make early determination of respectability possible, to be better for complete peri-SMA lymph nodes dissection, and to cause less operative bleeding despite longer operation time. Other options for approach to SMA have been introduced with potential indications, but any good evidences are not available yet [4].
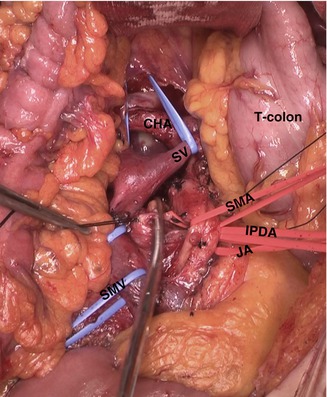

Fig. 23.8
Operative field of mesenteric approach (T-colon transverse colon, SMA superior mesenteric artery, SMV superior mesenteric vein, JA jejunal artery, IPDA inferior pancreaticoduodenal artery, SV splenic vein)
SMA-last approach is a conventional approach that is more familiar to most surgeons because it is simpler and easier compared to SMA-first approach and it has been working well for most of the periampullary cancers including pancreatic head cancer. So unless pancreatic cancer is located, uncinate process or major vascular invasion – at least abutting – is suspected, and SMA-last approach can be chosen.
If PV/SMV invasion is suspicious and combined vein resection is expected, vein resection should be the last procedure for en bloc removal of the specimen. To make it possible, uncinate process should be divided from SMA first before vein resection (Fig. 23.9).
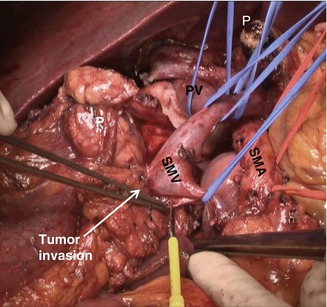

Fig. 23.9
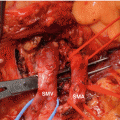
Operative field view just before en bloc removal of specimen in pancreatic head cancer with SMV invasion (P pancreas, SMA superior mesenteric artery, SMV superior mesenteric vein, PV portal vein)
23.1.4 Design for Portal Vein Resection
Surgeons should design portal vein resection for those who have suspicious portal vein invasion. Whether wedge resection or segmental resection with or without different types of graft is chosen should be determined according to the site and the extent of invasion. And the order of vessel dissection should be customized as described above. For instance, if there is no vein invasion, SMV can be separated from pancreatic head first, and if invasion is suspected, SMA first with SMV last is recommended for en bloc removal of specimen. Graft that could be used is diverse, autograft, allograft, or xenograft. There are different sources of autograft such as the left renal vein, jugular vein, external iliac vein, saphenous vein, etc. Frozen vessels from organ donor can be used and bovine patch grafts are used.
All the reconstruction procedures are to aim preserving portal blood flow as much as possible. Splenic vein and inferior mesenteric vein are recommended to be preserved unless vascular invasion is suspected. Some surgeons prefer splenic vein cutting for better exposure of the superior mesenteric artery and retroperitoneum (more for vascular resection and reconstruction is covered in other chapters).
23.1.5 Extent of Lymph Node Dissection
Lymph node metastasis has been known strong prognostic factor of pancreatic cancer, and extensive dissection of regional lymph nodes has been considered to bring some survival benefit. To investigate whether extensive lymph node dissection has any beneficial effect on survival in pancreatic head cancer, five randomized controlled trials have been tried to compare between limited LN dissection and extended LN dissection [5–9]. Although there were slight differences in the extent of LN resection (Fig. 23.10 and Table 23.1), all the studies failed to show any survival benefit of extended dissection. Therefore, it has been documented that standard or limited LN dissection is recommended (more for extent of surgery is discussed in other chapters). However, the results of these RCTs have not made most surgeons routinely perform standard limited LN dissection for pancreatic head cancer. Actually in most institutions, the extent of PD is customized between standard PD and extended PD.
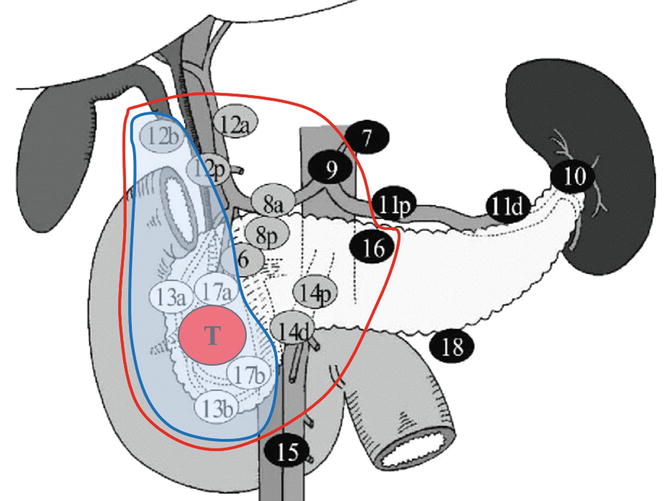

Fig. 23.10
Regional LN for pancreatic head cancer. The figure shows the definition of the extent of LN resection in the author’s study, standard (blue lined area) and extended (red lined area) dissection
Table 23.1
Extent of LN dissection in reported RCTs: standard PD vs extended PD
Standard/Extended | Pedrazzoli (Italy, 1998) | Yeo (JHI-USA, 2002) | Nimura (Japan, 2004) | Farnell (Mayo-USA, 2005) | Jang (Korea, 2013) |
|---|---|---|---|---|---|
Extent of LN dissection | 8, 13, 17 vs +9, 12, 14, 16 | Regional vs +perigastric, 16a2+b1 | 13, 17 vs + 8, 9, 12, 14, 16 | 12bc,13, 14ab vs +8, 9, 12a, 14cd, 16a, 2b | 12b, c, 13, 17 vs + 8, 9, 12a, 14, 16a2, b1 |
Retrieved LN | 13.3/19.8 | 17/28.5 | 13.3/40.1 | 15/36 | 17.3/33.7 |
For customizing LN dissection, several points should be considered. For R0, LN site of frequent metastasis and recurrence should be considered. There should not be additional morbidity. LN dissection can be done for biopsy purpose. Prognosis of cases with direct LN invasion has been reported better than that of cases with typical LN metastasis [10]. So LN dissection can be customized according to the tumor location.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree