(1)
Daytona Beach Shores, FL, USA
Some see a hopeless end, while others see an endless hope.
– Unknown
12.1 From the Patient’s Perspective
12.1.1 First the Basics
Facing a diagnosis of cancer can be psychologically devastating. The state of mind of most patients facing a catastrophic life event evolves through five stages: denial, anger, bargaining, depression, and acceptance [635]. In the denial phase, patients’ reaction often is “this can’t be happening, not to me”, which evolves to “why me? It’s not fair” characteristic of the anger phase. Then comes the “I’ll give anything for…” of the bargaining stage, followed by the “why bother” of the depression phase, shifting to “It’s going to be OK” of the acceptance phase. In cancer patients, the latter phase is often translated into a resolute determination to “fight” and “beat” the disease, which culminates in inner peace and the acceptance of death when it becomes clear that treatment has failed. Thus, it is not surprising that most patients opt for treatment: any treatment that offers some hope. This forward-looking fighting spirit, anchored on the primeval human instinct of self-preservation, often is bolstered by a subjective understanding of information disclosed by the physician, retaining positive elements while misinterpreting or unconsciously dismissing negative ones. Hence, in the US and some Western societies where “breaking bad news” has become an accepted practice, the physician must provide hope and emotional healing throughout the disclosure process. This can be accomplished by ensuring that the patient is ready to assimilate bad news, that information provided meets the patient’s wants, needs and preferences, and that the emotional impact of bad news is mitigated or retrieved by emphasizing whatever positive aspects of the case. Factors that can affect the patient’s understanding benefits and risks of treatment should be taken into account. They include the disclosure venue and timing (hospital, office, and context settings); the content of the disclosure (thoroughness, clarity, and specificity); and the level of personal interest and empathy conveyed by the physician [636]. Only then should the Oncologist formulate a management plan, taking into account the biological, psychological, behavioral, and social aspects of the patient’s disease and his/her input [637, 638]. The founder of the Schwartz Center movingly described his own experience with lung cancer only days before his death to illustrate the enormous power of caregivers’ empathy on a patient’s frame of mind [639].
In many regions of the World, physicians censor the information shared with cancer patients in misguided attempts to protect them from the potential emotional harm of bad news [640–642]. Sheltering patients from bad news has had unintended consequences. It increases fear and anxiety, prevents a trusting physician-patient relationship to develop, and deprives patients from the empowering feeling of actively participating in the decision-making process and ongoing care. In societies where disclosing bad news is accepted practice, patients often control the type and amount of medical information disclosed to them depending on their level of anxiety, ability to cope, and other personal factors. For example, while some patients demand full disclosure in order to actively participate in their own care, patients with the greatest fear of death and a perception of poor prognosis intuitively prefer minimal disclosure, relinquishing all decisions to the physician [643]. In the end, most cancer patients defer to their physicians the choice of therapy, especially when treatment recommendations are presented with empathy but self-assurance. The preceding general guidelines ensure appropriate physician-patient communications, safeguard patients rights, and enable treatment plans that respond to patients’ needs and wishes. Special guidelines apply to clinical trials and are regulated by Federal laws requiring all human research to be conducted according to basic ethical principles that ensure respect for the individual, beneficence, and justice, and that patient participation be informed, voluntary, and not influenced or coerced in any way.
12.1.2 More Details
12.1.2.1 Disclosure in the Community Setting
It is stating the obvious to assert that the Oncologist planning treatment and the cancer patient being advised should both be aware of the risks and benefits of the recommended treatment and have a clear understanding of potential outcomes. Thus, it might be expected that a substantial number of patients afflicted by advanced cancer of the types proven over the years to follow a relentless course not substantially altered by treatment would not be offered treatment or decline it if offered. However, the vast majority of cancer patients are treated despite the fact that fewer than 2 % achieve a cure and a meager prolongation of survival is the best hope [644]. This is due not only to Oncologists’ pro-treatment stance, but also to patients’ attitudes regarding cancer treatment. Indeed, studies have shown that cancer patients are willing to assume greater risks when facing imminent death than would caregivers and the public. According to one survey, 53.1 % of cancer patients expressed a willingness to suffer severe treatment side effects in order to reach a 1 % cure rate, 42.1 % to survive an additional 3 months, and 42.6 % to achieve symptom relief, compared to 20, 10.2, and 6.8 % of Oncologists, 13.5, 6.0, and 5.9 % of Oncology nurses, and 19, 10, and 10 % of healthy controls [645]. Is this risk-taking attitude by cancer patients a rational, weighted decision? Is it the result of over-enthusiastic physicians emphasizing small benefits while minimizing risks? Or, is it the result of desperate decisions of individuals in despair when confronting imminent death? In most instances, patients’ role in the decision-making process is limited to acquiescing to the physician’s choice of action after asking but a few questions, despite surveys indicating that most patients in all age groups prefer to participate actively in decision-making [646, 647]. Indeed, not only do the psychological and emotional impacts of a cancer diagnosis diminish patients’ analytical power and discerning capacity at a time it is most needed, but most patients make no attempts to research their disease or treatment options independently. Given the circumstances, the instinct of self-preservation usually prevails, leading most patients to hear what they wish to hear, particularly if the content and setting of the disclosure process and empathy of the caregiver are sub-optimal [648, 649].
Trust in the physician is an essential element of the patient-physician relationship. However, a physician becomes a perfect agent for the patient only when the latter would make the same decision if in possession of the same clinical information and expertise. Such a circumstance rarely exists in practice, for few patients will ever achieve a level of understanding comparable to that of their physicians, and the latter often fail to meet patients halfway [650]. In practice, multiple Oncologist-patient encounters will take place over the course of the disease, involving three stages each time: exchange of information, discussion, and decision-making. The type of interaction depends on the patient-physician relationship, which generally takes one of three forms [651]. The first is the traditional paternalistic model, where information flows in one direction: from physician to patient, all decisions regarding treatment and patient management are made by the physician, and the patient acquiesces to professional authority and expertise. At the other extreme lies the informed model where information flows from physician to patient but the patient makes all decisions. Between these extremes is the shared model where exchange of information proceeds both ways: the physician thoroughly informs the patient of treatment options along with their benefits and risks, and the patient voices preferences, and both contribute to the decision-making process. While by virtue of their training and expertise, and for expediency, most physicians tend to adopt the paternalistic approach, patients’ preferences vary according to age, sex, educational level, and type and severity of the disease. For example, a study of 1,012 women with breast cancer revealed that 22 % wanted to select their own treatment, 44 % elected to share the task with their physician, and 34 % preferred to delegate the responsibility to their physician [652]. Additionally, many patients are receptive to substantial amounts of information, even if they do not wish to participate in making treatment decisions. Under the shared model, patients must be in possession of substantial clinical information in order to participate effectively in treatment decisions that will profoundly impact their lives and often determine their survival. Yet, because communication skills are not taught in medical schools and there are no standard guidelines, the physician disclosure process is often inadequate, not geared to patients’ needs and preferences, and often overestimates benefits and underestimates risks. A good point of departure is to practice patient-centered care, defined as “respecting and responding to patients’ wants, needs and preferences, so that they can make choices in their care that best fit their individual circumstances”, as the cornerstone of quality care delivery [653].
It might surprise the reader to know that there are no specific guidelines for cancer care, though generalities have been addressed over the years. For instance, in 1999, the Institute of Medicine (IOM) concluded that cancer patients did not receive state-of-the-art care and its National Cancer Policy Board Cancer Care System made 10 recommendations for promoting optimal care delivery. While reasonable, the measures recommended are broad and designed to become pubic policy rather than apply to individual care. For instance,
Yet, none address the initial physician-patient phase that sets the stage for a mutually satisfying relationship and in large measure determines a seamless course of action and peace of mind for the patient. There is consensus that this relationship should rest on a transparent disclosure of diagnosis, proposed intervention with reasonable alternatives, intended benefits, and associated risks. However, there are no specific guidelines addressing when to initiate treatment towards what outcome and whether to discontinue treatment when it becomes clear that the intended outcome cannot be reached or when the burden of treatment becomes unacceptable. What Oncologists must correct is the widespread practice of assessing treatment efficacy based on tumor response rather than on patient outcome, which explains in large measure why cancers historically shown to progress relentlessly regardless of treatment are routinely treated, often to the end of life. Indeed, potential benefits of cancer treatment in the clinical setting should be measured not by tumor responses but by patient outcome benchmarks, including OS, 5-year survival, and DFS. Such information is readily available from the medical literature for most cancers in all stages and should be used as the sole guide for deciding whether or not chemotherapy will be of benefit to a particular patient, and so inform the patient. Tumor outcomes, such as average response rates and partial or complete remissions gathered from the literature, should be discussed. However, physicians must inform patients that the latter represent not goals in themselves but interim measurements of tumor size that are useful for determining whether the therapy instituted is efficacious and worth pursuing or inefficacious and should be abandoned. Physicians should stress that tumor responses have little relevance to the ultimate course of the disease or to patient survival. Likewise, potential complications, especially life-threatening toxicity of the treatment contemplated, and their management, should also be disclosed at the outset. Alternative treatments, including withholding or delaying treatment, and the effect of each on survival and QOL, should be discussed. Likewise, because pain is one of the most feared complications of cancer, patients must be reassured that pain control is easy to achieve in most circumstances given today’s potent analgesics, and physicians should not flinch from prescribing opioids when appropriate [658]. Finally, patients should be encouraged to ask questions, to discuss their options with loved-ones or seek second opinions, and be allowed time for reflection. Oncologists who adopt this advisory role are rewarded by enlightened patients who tend to become participants in their own care, and who have a greater appreciation of the highly complex issues involved in cancer management and, having understood and accepted the risks involved, are less likely to resort to unwarranted legal action.
On the other hand, ASCO’s “Measures of Quality of Care for Patients With Cancer” recommends “The selection and proper application by clinicians of clinical treatments that optimize the outcomes of care” as part of the “initial therapeutic management” [655]. Under its Quality Oncology Practice Initiative, ASCO lists 97 items with subdivisions, all of which are very general, such as “core 9: Documented plan for chemotherapy, including doses, route, and time intervals.” Or “core 13a1: Chemotherapy administered to patients with metastatic solid tumor with performance status of 3, 4, or undocumented (Lower Score – Better)” [656]. Likewise, the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology lists the following specific guidelines:
Recommendation 3: Measure and monitor the quality of care using a core set of quality measures. Cancer care quality measures should span the continuum of cancer care and be developed through a coordinated public-private effort: be used to hold providers, including health care systems, health plans, and physicians, accountable for demonstrating that they provide and improve quality of care; be applied to care provided through the Medicare and Medicaid programs as a requirement of participation in these programs; and be disseminated widely and communicated to purchasers, providers, consumer organizations, individuals with cancer, policy makers, and health services researchers, in a form that is relevant and useful for health care decision-making [654].
For treatment of cancer by site.
For detection, prevention, & risk reduction.
For supportive care.
For age-related recommendations.
For patients [657].
All factual information described above should be recorded on a written informed consent form that could follow recommendations by the Office of Human Research Protections for clinical trials [659]. The form, to be written in easy to understand language, should prominently display the following information:
Short text or tabular description of the patient’s diagnosis (e.g., cancer type – stage), and special circumstances of the case likely to skew expected outcome.
Best drug or drug combination recommended, plus one or two alternative options.
Anticipated patient outcome (e.g., DFS – 5-year survival) and risks associated with each drug option, stratified by severity and probability of occurrence.
Summary of pertinent medical literature data on benefits and risks of treatment options recommended, along with the actual references.
Disclosure of patient rights, including well-being and privacy protection, and autonomy.
Description of patient-triggered issues and other subjects that were discussed.
Disclosure of the physician’s financial interest in drugs and tests recommended.
In order to streamline and standardize the process, inform consent forms could be developed for the ten most frequent cancers (e.g., prostate, breast, lung, colorectal, melanoma, bladder, lymphoma, kidney, thyroid, and pancreas) that accounted for 74.8 % of all cancers in 2012. Alternatively, the ten most lethal cancers could be targeted initially (e.g., lung, colorectal, breast, pancreas, prostate, lymphoma. ovary, esophagus, bladder, and brain), which accounted for 66.2 % of all cancer deaths in 2012. The task of designing such specific consent forms could be assigned to panels of experts from NCI, ASCO, ASH, cancer cooperative groups, universities, or research centers and, after securing input by the Medical Oncology community, be made freely available on the Web to anyone. Some will argue, correctly, that such a document cannot apply to all situations. However, sufficient flexibility could be built into the document to allow timely modifications according to the individual’s disease and psychosocial profiles, with the added advantage that each would be discussed with the patient and recorded as rationale for treatment adjustments. There is precedent for this in clinical trial protocols where initial and subsequent adjustments to the treatment proposed are allowed in order to individualize care and reduce risks. Moreover, the expanded information disclosure process and informed consent form would codify, organize, and bring uniformity, transparency, and objectivity to a process that many community Oncologists follow in principle, but in an inconsistent and poorly-documented manner. Additionally, a thorough, transparent, and objective initial disclosure would serve as a basis for future physician-patient communications and facilitate subsequent management decisions as they become necessary during the course of the disease. This approach is applicable to all cancer patients, including those who choose a “paternalistic” physician-patient relationship model and prefer to be in possession of rudimentary rather than detailed information about their disease and its treatment. In cases of extreme reluctance, detailed information can be conveyed primarily to the patient’s surrogates. By providing written evidence that patient and physician reviewed the case management specifics together, a signed consent form of this nature would encourage and generalize the practice by allaying physicians’ fears of litigation and ultimately be helpful in court cases.
12.1.2.2 Disclosure Within Clinical Trials
The first international codification of ethical principles to guide clinical researchers involved in human experimentation is known as The Declaration of Helsinki. This document entitled Ethical Principles for Medical Research Involving Human Subjects was adopted by the 18th World Medical Association (WMA) general assembly meeting in Helsinki, in June 1964. The 9th of its 12 Basic Principles reads,
The declaration was first revised in 1975 at the Tokyo 29th WMA meeting where the concept of Independent Review Boards was introduced, with the seventh and last revision being in 2008 at the 59th meeting in Seoul, which codified registration of clinical trials and reporting of results. The final document is universally regarded as the ethical cornerstone of human clinical research.
In any research on human beings, each potential subject must be adequately informed of the aims, methods, anticipated benefits and potential hazards of the study, and the discomfort it may entail. He or she should be informed that he or she is at liberty to abstain from participation in the study, and that he or she is free to withdraw his or her consent to participation at any time. The physician should then obtain the subject’s freely-given informed consent, preferably in writing [660].
In the US, codification of human research began with the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research issued by the National Research Act signed into law on July 12, 1974. Among other tasks, the commission was charged with identifying the basic ethical principles that should underlie the conduct of biomedical and behavioral research involving human subjects, and to develop guidelines which should be followed to assure that such research is conducted in accordance with those principles. The commission first met on February 1976 at the Smithsonian Institution’s Belmont Conference Center, followed by monthly deliberations over a period of nearly 4 years, which resulted in a Department of Health and Human Services’ publication entitled: The Belmont Report: Ethical principles and guidelines for the protection of human subjects of research, best known as the Belmont Report [661].
The Belmont Report expanded the Nuremberg Code [662], drafted in 1947 as a set of standards for judging researchers who conducted unethical biomedical experiments on concentration camp prisoners during WWII, and was designed to prevent repetition of medical research abuses revealed in 1972 regarding the natural history of untreated syphilis [663]. This referred to a study conducted in the 1940s on 399 poor black males from Tuskegee, Alabama who were not informed of their disease (syphilis) and denied penicillin when it became available in 1947, leading to a full enquiry and an apology by President Clinton in 1997. Other infamous unethical experiments, highly implausible today thanks to legislation enacted since, came to light in a 1993 exposé by The Albuquerque Tribune. It revealed both the injection of radioactive tracers to thousands of unsuspecting human subjects and the radiation exposure of thousands of unaware individuals to several hundred secret and intentional releases of radiation over a 30-year period. Half a century later, on January 15, 1994, President Clinton created the Advisory Committee on Human Radiation Experiments to investigate reports of unethical conduct by the US government and by government-funded institutions, in the use of, or exposure to, ionizing radiation in human beings during the period of 1944–1974. In its final report, the panel described the following illustrative case:
The subject, as it turned out, was already in the Oak Ridge Army hospital, a victim of an auto accident that had occurred on March 24, 1945. He was a fifty-three-year-old “colored male” named Ebb Cade, who was employed by an Oak Ridge construction company as a cement mixer. The subject had serious fractures in his arm and leg, but was otherwise “well developed [and] well nourished.” The patient was able to tell his doctors that he had always been in good health. Mr. Cade had been hospitalized since his accident, but the plutonium injection did not take place until April 10. On this date, “HP-12” (the code name HP – “human product”)…was reportedly injected with 4.7 micrograms of plutonium… Measurements were to be taken from samples of Mr. Cade’s blood after four hours, his bone tissue after ninety-six hours, and his bodily excretions for forty to sixty days thereafter. His broken bones were not set until April 15-five days after the injection-when bone samples were taken in a biopsy…One document records that Mr. Cade had “marked” tooth decay and gum inflammation, and fifteen of his teeth were extracted and sampled for plutonium. The Committee has not been able to determine whether the teeth were extracted primarily for medical reasons or for the purpose of sampling for plutonium…According to one account, Mr. Cade departed suddenly from the hospital on his own initiative… [664].
From the Belmont Report and subsequent legislative efforts was born the modern informed consent, a document that establishes three fundamental ethical principles that must be observed in any human research:
Several reviews of the content and scope of the informed consent have been published over the years, including some quite recently [665]. The oversight and responsibility for implementing guidelines for experimentation in humans rests with the IRB at centers that conduct human research [666]. On the other hand, uniformity of the disclosure process is ensured by the informed consent, which informs and engages patients, and sets the stage for open and transparent communications between patient and physician for the duration of the study. However, the Belmont Report and a structured informed consent protect the approximately 2 % of the total US cancer population participating in clinical trials at any given time, but do not apply to the remaining 98 %, who receive non-investigational cancer treatment in the community setting [667–669]. In addition, critics contend that social or mind control experimentation are not covered. Another loophole is the use of cancer drugs off-label. This practice consists of using drugs for indications other than those approved by the FDA or in combinations not sanctioned by prior clinical research, especially for treating patients who have failed standard treatment. Yet, this is permitted by the Belmont Report in which “practice” is defined as “interventions that are designed solely to enhance the well-being of an individual patient or client and that have a reasonable expectation of success.” In contrast, research is defined as “an activity designed to test an hypothesis… described in a formal protocol that sets forth an objective and a set of procedures designed to reach that objective.” Although the use of cancer drugs off-label might be justified occasionally by seasoned Oncologists’ breadth and depth of knowledge, the practice is widespread and often based on anecdotal personal experience rather than on clinical trial data. As a result, unsuspecting patients so treated are exposed to additional and often unforeseen side effects and complications without commensurate benefits. While most Oncologists are judicious in the use of drugs off-label, the unrestricted practice could theoretically extend to any drug and any cancer.
Respect to persons by acknowledging participants’ autonomy and protection for those with diminished autonomy.
Beneficence that ensures that no harm will be done and to maximize possible benefits and minimize possible harms.
Justice, the principle of shared burdens and benefits, to ensure that potential benefits of the research benefits study participants as well as non-participants.
The quality of the disclosure, or lack thereof, can also be at issue, both in clinical trials and in the clinical setting. Audio- or video-taped surveys of Oncologists’ interviews with prospective clinical trial participants demonstrated multiple deficiencies in the disclosure process that, in some cases, calls into question the validity of the informed consent [670, 671]. Indeed, while most Oncologists are conscientious and dedicated to their patients’ welfare, the desire to communicate in simple language, poor communication skills, and time constraints often lead to insufficient or faulty disclosure. Alternatively, the zeal for thoroughness might result in disclosure of superfluous and confusing details, or the use of an overly complicated or technical language not readily comprehensible by some patients. Recent advances in research technologies often generate incidental findings, raising the issue of whether researchers and caregivers should disclose such information to patients when non-essential to the clinical study or to the treatment contemplated [672]. However, more troubling, though less common, is the conduct of some unscrupulous researchers who disregard ethical principles and violate Federal rules designed to protect research subjects for expediency or self-serving motives, putting patients at risk and exposing themselves to FDA censure, as in the case of an editor of the NEJM [673], loss of Federal research funding and academic standing [674, 675], humiliating retraction of published data [676], dismissal [677], or lawsuits [678]. More ominously, each new revelation of unethical conduct by medical researchers contributes to public distrust in clinical research and raises questions as to whether they represent isolated cases or the tip of an iceberg of research misconduct.
12.2 From the Caregiver’s Perspective
12.2.1 Facing Difficult Decisions
Approximately 2 % of patients with disseminated or metastatic cancer treated with chemotherapy can be cured of their disease, and marginal to modest prolongation of survival, usually measured in weeks, is feasible in some types of cancer [679]. Hence, treating the vast majority of patients with advanced cancer unless contraindicated, as is the practice today, would seem to be an unwise if not futile exercise. Indeed, when the most desirable “quantitative” patient outcomes such as cure, 5-year survival, or meaningful prolongation of survival are not achievable, seeking “qualitative” outcomes such as palliation and improved QOL become the Oncologist’s ultimate goal. While the above statement is intuitively obvious and unassailable, it embodies concepts and issues that, given their ethical, social, and legal context, are difficult to sort out in the clinical arena. For example,
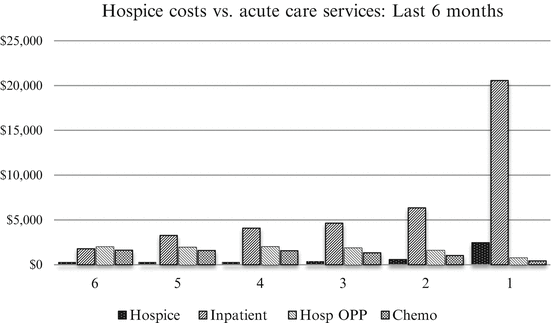
The above questions and many others raise issues Oncologists are confronted with on a daily basis that encompass religious, moral, ethical, and socioeconomic factors, in addition to medical considerations. The sequence of the above list rests on the fact that the concept of palliation, its modality, and delivery setting are most pertinent and crucial in the context of terminal patients when treatment of the disease has failed and the physician must focus on ensuring the best possible QOL during the patient’s final stages. Hence, the pivotal decision to initiate the palliative phase is triggered by the determination that further disease-specific treatment would be futile. Yet, the absence of guidelines as to timing and the controversy regarding rights of autonomy from the caregiver’s and patient’s perspectives and the definition of medical futility raise new questions. On the one hand, modern medical science enables physicians to maintain and prolong vegetative states through mechanical ventilation, feeding tubes, and other life-supporting means, and patients and patient surrogates increasingly assert their autonomy in life and death decisions, often with a limited understanding of the consequences. On the other hand, the concept of medical futility is misunderstood by the various stakeholders in its scope and timing. For instance, a survey of surrogates of 50 critically ill hospitalized patients revealed that most doubted the ability of physicians to predict medical futility [680]. Likewise, a Medline database search of studies on medical futility between 1980 and 2008 revealed that 47 studies supporting and 45 refuting the concept of medical futility provided insufficient clinical guidance for making such a decision [681]. Moreover, while the concept of QOL is intuitively obvious, it is neither definable nor quantifiable with any degree of consensus. More ominously, the closely related notion of palliation has two incompatible and widely divergent interpretations in the practice setting: one that properly views palliation in the context of alleviating suffering through symptom relief; the other that equates palliation with the administration of non-curative treatment, presumably aimed at controlling disease-related symptoms or, more unrealistically, at preventing future complications of progressive cancer. While the former addresses patients’ immediate QOL concerns, the latter justifies inflicting additional pain and suffering now in exchange for a usually unfulfilled promise of a better future QOL. This attitude towards palliation suggests that cancer caregivers, for a variety of personal and extraneous reasons, are unable or unwilling to acknowledge in a timely manner that their efforts to change the course of their patients’ cancer has come to an end. To do so should not be construed as abandonment but rather as a humane approach to prevent further suffering at the end of life by providing appropriate palliative care and an opportunity for patients and relatives to come to terms with the dying process and achieve peace and dignity. In contrast, terminal patients often receive more intensive care in the last few weeks of life than in the preceding months. This is documented yet again in a recent study of end-of-life health care costs incurred by 28,530 privately insured oncology patients between July 2002 and December 2009 [682]. In that study, the mean total cancer-related costs in the last 6 months before death was $74,212, of which 79 % was incurred for three types of acute services: Inpatient care, $40,702 or 55 %; Hospital outpatient procedures (Hosp. OPP), $10,123 or 13.6 %; and Chemotherapy, $7,595 or 10.2 %, with Hospice care accounting for only $3,256 or 4.4 % of the total. While on the sixth month before death, outpatient procedures, inpatient care, chemotherapy, and hospice cost $1,992, $1,785, $1630, and $28, respectively, hospice care cost rose to $2,464 in the last month of life, whereas inpatient care cost soared to $20,559 or nearly 85 % of total cost (Fig. 12.1).
When is a cancer refractory and its treatment futile, leading to palliation?
What is palliation?
Is palliative chemotherapy justified when efficacious alternatives are available?
How much chemotherapy toxicity is acceptable for what type and level of palliation?
Does the placebo effect of chemotherapy justify its use in palliation?
What is the preferred setting for providing optimal palliation?