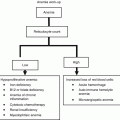
Points
Age (years)
ECOG performance status
LDH/ULN
Leukocytes (×109/L)
0
<50
0–1
<0.670
6,700
1
50–59
–
0.670–0.999
6,700–9,999
2
60–69
2–4
1.000–1.499
10,000–14,999
3
>69
–
>1.499
>14,999
Risk stratification | |
|---|---|
0–3 points | Low risk |
4–5 points | Intermediate risk |
6–11 points | High risk |
Minimal Residual Disease
PCR-based evaluation of MRD has shown remarkable predictive value in MCL. Starting from the first prognostic demonstrations in autologous transplantation (auto-SCT) field [44], the value of MRD as powerful independent outcome predictor was prospectively validated in two large European MCL Network trials [19]. Most notably the predictive value of MRD detection was observed both in young patients treated intensively and in elderly patients receiving conventional treatment and maintenance either with interferon-alpha or rituximab. Those patients who obtained a complete molecular response (defined as negativity of an allele-specific Real Time PCR analysis, having a minimal sensitivity of 1 neoplastic cell per 104 healthy cells) demonstrated a significantly longer remission duration versus those patients who did not obtain such MRD levels. The differences were impressive also for elderly patients: the 2-years rate of ongoing remissions differed from 76 to 36 % for patients with molecular remission versus non-molecular responsive patients, respectively. Thus, MRD negativity may be obtained also in elderly patients and the achievement of molecular remission is meaningful for the long-term outcome.
On the other hand the limitations to a widespread use of MRD analysis in the clinical practice are that MRD detection by PCR is not devoid of costs and should be only performed in centralized experienced laboratories, despite a considerable standardization effort is ongoing [45].
Based on the reliability of MRD detection in MCL, tailored treatment driven by PCR results has been employed, mainly targeting molecular relapses of autografted MCL patients [46–48]. Rituximab monotherapy led to re-induction of molecular remission in the majority of patients and, based on the larger Nordic study, seemed to provide clinical benefit, too [47]. However, the broad applicability of such approaches still needs to be proven, in particular among elderly patients receiving conventional treatment only.
Thus MRD detection, despite being a powerful independent predictor, is not yet recommended in clinical routine outside of clinical trials, due to its limitations of applicability, reproducibility and validation [34].
Current Diagnostic Standards
The diagnosis of MCL is established according to the criteria of the WHO classification of hematological neoplasms. In general histologic confirmation of diagnosis is mandatory and a lymph node biopsy is strongly recommended. In contrast, fine-needle biopsy is not appropriate. Bone marrow aspiration alone is not sufficient but should be complemented by flow cytometry to identify the typical lymphoma immunophenotype and bone marrow biopsy to quantify the percentage of infiltration [49]. Most tumours have a classic morphology of small-medium sized cells with irregular nuclei, dense chromatin, and unapparent nucleoli. In addition to classic MCL, a blastoid variant of this disease has been described that is characterized by high mitotic rate and particularly aggressive behaviour and is associated with INK4a/ARF deletions, TP53 mutations, and complex karyotypes [15, 32, 50, 51]. However, the tumour cells may present with a spectrum of morphological variants that may raise some difficulties in the differential diagnosis with chronic lymphocytic leukaemia, marginal zone lymphomas, large B-cell lymphomas, or blastic hematological proliferations. Because an accurate histologic diagnosis is essential, second opinion by an experienced hematopathologist is advisable [34].
Beside the classical immunophenotype (immunoglobulin M/D, CD19, CD20, CD22, CD43, CD79a, CD5 positive and CD23, CD10, CD200, BCL6 usually negative), the detection of cyclin D1 overexpression or the t(11;14) translocation is essential, since histo-morphological phenotypes may differ significantly [5, 15, 52, 53]. Nevertheless, rare cases of cyclin D1-negative variant of MCL has been recognized [54], characterized by the same gene expression profiling and genomic alterations as classical MCL and showing in 50 % of cases a cyclin D2 translocation [33, 55, 56]. SOX11, a transcription factor expressed in 90 % of MCL, may be applied to identify this variant [57, 58]. Moreover, as already stated, Ki67 proliferative index staining is recommended as a strong prognostic indicator of long term outcome [34].
The laboratory evaluation comprises differential blood count and standard serum chemistry analysis, including the determination of LDH as one of the major risk parameters. Abdominal CT of the neck, chest, abdomen, and pelvis is mandatory. Cerebrospinal fluid evaluation and cranial imaging with MRI is not usually required at first presentation, unless neurologic symptoms are present [3]. PET scan is not included in the consensus recommendations based on scarce data and especially limited therapeutic consequences [59–62]. Additional diagnostics depends on the clinical presentation and includes an ear-nose-throat consultation and gastroscopy/colonoscopy, based on up to 60 % asymptomatic infiltration of the bowel [63, 64]. As the results from upper and lower endoscopy have generally only a modest impact on therapeutic decisions, they are mandatory only in limited stages symptomatic patients [64] or as confirmation of complete responses within clinical trials [65].
Current Treatment Concepts
Given the high median age of MCL patients and considering that no curative treatment is available so far, toxicity of induction therapy is a major concern. Thus, it is essential to define the therapeutic goal, which may be long term control of the disease, balancing the expected treatment efficacy (remarkable lifespan prolongation) against the risk of impairing toxicity and reduced quality of life. Consequently, it may be important to obtain a complete response (CR) also in elderly patients. Given that a good performance status and the absence of comorbidities are required for any intensified treatment aiming at CR, a common approach consists of an upfront stratification of patients into elderly fit and elderly frail categories [68]. Comprehensive Geriatric Assessment (CGA) was demonstrated as a reliable tool to objectively identify patients eligible for a chemotherapy targeting at long term control of the disease or patients for palliative approaches only [69–71].
A summary of the most important recently published clinical studies involving elderly MCL patients is shown in Table 9.2.
Table 2
Recent published clinical studies involving elderly MCL patients
Author | Study features | Evaluable MCL patients | Therapeutic regimen | ORR% (CR%) | Median PFS (months) | Median OS (months) | |
|---|---|---|---|---|---|---|---|
Conventional immuno chemotherapy | Kluin-Nelemans et al. (2012) [79] | Phase III, first-line, randomized | 485 | Induction: R-CHOP vs R-FC | 86 (34) vs 78 (40) | 28 vs 26 (TTF) | 62 % vs 47 % (4-year OS) |
Maintenance: rituximab vs interferon alpha | – | 58 % vs 29 % (4-year DOR) | 79 % vs 67 % (4-year OS) | ||||
Rummel et al. (2013) [82] | Phase III, first-line, randomized | 94 | R-CHOP vs rituximab-bendamustine | na | 21.1 vs 35.4 | na | |
Visco et al. (2013) [84] | Phase II first-line/relapse | 40 | Rituximab. bendamustine. cytarabine | 90 (83) | First-line 95 % relapse 70 % (2-y PFS) | na | |
Proteasome inhibitors | Ruan et al. (2010) [90] | Phase II, first-line | 36 | R-CHOP + bortezomib | 91 (72) | 44 % (2-year PFS) | 86 % (2-year OS) |
Houot et al. (2012) [91] | Phase II, first-line | 39 | Rituximab. doxorubicin, dexamethasone, chlorambucil, bortezomib | 79 (59) | 26 | 69 % (2-year OS) | |
Goy et al. (2009) [85] | Phase II, relapse | 141 | Bortezomib | 33 (8) | 6.7 (TTP) | 23.5 | |
Lamm et al. (2011) [104] | Phase II, relapse | 16 | Bortezomib, rituximab, dexamethasone | 81 (44) | 12.1 | 38.6 | |
Kouroukis et al. (2011) [108] | Phase II, relapse | 25 | Bortezomib, gemcitabine | 60 (11) | 11.4 | na | |
mTOR inhibitors | Hess et al. (2009) [109] | Phase III, relapse, randomized | 54 | Temsirolimus 175/75 | 22 (2) | 4.8 | 12.8 |
54 | Temsirolimus 175/25 | 6 (0) | 3.4 | 10 | |||
53 | Investigator’s choice | 2 (2) | 1.9 | 9.7 | |||
Ansell et al. (2011) [110, 111] | Phase II, relapse | 69 | Temsirolimus. rituximab | 59 (19) | 9.7 | 29.5 | |
Renner et al. (2012) [112] | Phase II, relapse | 35 | Everolimus | 20 (6) | 5.5 | na | |
Immunomodulatory drugs | Witzig et al. (2011) [89] | Phase II, relapse | 57 | Lenalidomide | 42 (21) | 5.7 | na |
Eve et al. (2012) [113] | Phase II, relapse | 26 | Lenalidomide | 31 (8) | 3.9 | 10 | |
Wang et al. (2012) [114] | Phase II, relapse | 44 | Lenalidomide, rituximab | 57 (36) | 11.1 | 24.3 | |
Zaja et al. (2012) | Phase II, relapse | 33 | Lenalidomide, dexamethasone | 52 (24) | 12 | 20 | |
Harel et al. (2010) [116] | retrospective, relapse | 58 | Thalidomide ± bortezomib ± rituximab | 50 (21) | 29 % (1-year TTF) | 62 % (1-year OS) | |
Ruan et al. (2010) [101] | Phase II, relapse | 22 | RT-PEPC | 73 (32) | 10 | 45 % (2-year OS) | |
Antibody-based approaches | Smith et al. (2012) [95] | Phase II. first-line | 50 | R-CHOP + 90γ-ibriturnurnab tiuxetan | 64 (46) | 30.8 (TTF) | 73 % (5-year OS) |
Wang et al. (2009) [118] | Phase II. relapse | 32 | 90γ-ibritumumab tiuxetan | 31 (16) | 6 (EFS) | 21 | |
Cartron et al. (2010) [122] | Phase II. relapse | 15 | GA-101 | 27 (na) | 2.5 | na | |
B-cell receptor signalling inhibitors and other targeted approaches | Wang et al. (2012) [120] | Phase II, relapse | 110 | Ibrutinib | 68 (22) | 13.9 | na |
Kahl et al. (2010) [121] | Phase I, relapse | 16 | Cal-101 | 62 (na) | 3 (DOR) | na | |
Lin et al. (2010) [128] | Phase I, first-line/relapse | 10 | Flavopiridol, fludarabine, rituximab | 80 (70) | 21.9 | na | |
Evens et al. (2012) [132] | Phase II, relapse | 11 | Abexinostat | 27 (na) | 4 | na |
First-Line Treatment of Elderly Fit Patients
Conventional Immunochemotherapy
Thus far, the most frequently applied chemotherapy schedules for elderly fit patients consist of 3-weekly R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) or R-CVP (rituximab, cyclophosphamide, vincristine and prednisone) depending on cardiac comorbidities [1, 65]. The anti-CD20 monoclonal antibody rituximab should always be added to induction therapy, as it results in improvement of overall response rate (ORR) and even OS in the absence of significant toxicity [14, 65, 72, 73].
Alternatively, fludarabine-containing schemes have been explored as well achieving high response rates, with or without cyclophosphamide and mitoxantrone (FC or FCM) [74–78]. On these basis the European MCL Network conducted a large international phase III trial comparing R-CHOP with R-FC (followed by a second randomization between maintenance phase with interferon alpha versus rituximab) for elderly patients. Unexpectedly, the outcome of the fludarabine-containing regimen was disappointing and accordingly this trial established R-CHOP immunochemotherapy followed by rituximab maintenance as the “gold standard” first-line therapy for elderly MCL [79]. In fact, although CR rates after R-FC and R-CHOP were similar (40 % versus 34 %), progressive disease was more frequent during R-FC (14 % versus 5 %). The median OS was also significantly inferior after R-FC (4-year survival rate, 47 % vs. 62 %) and more patients in the experimental arm died due to lymphoma or infections. In addition to lower efficacy, haematologic grade 3–4 toxicity was more frequent during R-FC. Thus, the use of upfront R-FC in elderly MCL patients is discouraged [34].
Alternative chemotherapy regimens have been also explored. An important candidate is bendamustine, showing excellent responses in patients with relapsed MCL [80, 81]. Notably, in a randomized trial with 94 MCL patients, the schema rituximab-bendamustine (BR) compared favourably with R-CHOP in first-line treatment, achieving a prolonged progression-free survival (PFS: 35.4 vs 22.1 months) and fewer toxic effects (lower neutropenia, infections, polyneuropathy and alopecia) [82]. Bendamustine also performed well in first-line treatment of MCL if combined with vincristine and prednisone (BOP regimen) [83]. Moreover, the promising activity of a new regimen combining rituximab, bendamustine and cytarabine (R-BAC) has been recently confirmed in primary and relapsed MCL (90 % ORR with 83 % CR on the total series of 40 patients), resulting in an excellent 2-years PFS of 70 % for relapsed and 95 % for first-line patients, respectively [84]. Currently, a phase II study of R-BAC accruing untreated elderly “fit” (according to CGA) MCL patients is ongoing (EudraCT Number: 2011-005739-23, Table 9.3).
Table 3
Ongoing clinical trials accruing elderly MCL patients
NCT code | Study features | Estimated enrollment (patients) | Estimated primarycompletion date (month/year) | Therapeutic regimen | Sponsor | Location countries |
|---|---|---|---|---|---|---|
EudraCT Number: 2012-002542-20 | Phase III, randomized, first-line | 633 | 06/2019 | R-CHOP vs R-CHOP/R-HAD + maintenance rituximab vs rituximab/lenalidomide | LYSARC & EuMCLNet | France, Belgium Germany, Italy, Netherlands, Portugal |
01776840 | Phase III, randomized, first-line | 520 | 03/2018 | BR vs BR + ibrutinib | Janssen Research & Development LLC | Worldwide |
01415752 | Phase II, randomized, first-line | 332 | 04/2015 | BR ± bortezomib + rituximab maintenance ± lenalidomide | ECOG | USA |
01662050 | Phase II, single arm, first-line | 57 | 01/2014 | R-BAC | FIL | Italy |
00963534 | Phase II, single arm, first-line | 60 | 09/2014 | BR + lenalidomide | NLG | Denmark, Finland, Norway, Sweden |
01449344 | Phase III, randomized, relapse | 175 | 09/2016 | R-HAD vs R-HADB | EuMCLNet | France, Germany |
01646021 | Phase III, randomized, relapse | 280 | 08/2014 | Ibrutinib vs temsirolimus | Janssen Research & Development LLC | Worldwide |
01078142 | Phase I/II, single arm, relapse | 72 | 03/2014 | BERT | GLSG & EuMCLNet | Germany |
01389427 | Phase I/II, single group assignment, relapse | 63 | 06/2013 | R-CHOP or R-FC or R-HAD + temsirolimus | GOELAMS | France |
01737177 | Phase II, single arm, relapse | 42 | 07/2014 | R-2B | FIL | Italy |
New Targeted Drugs Combinations
Other promising candidates for combination with immunochemotherapy are bortezomib and lenalidomide, both effective as single agents in relapse setting [85–89]. R-CHOP combined with bortezomib achieved highly promising response rates in a phase II study of 36 primary MCL patients (ORR 91 % with 72 % CR/unconfirmed CR and a 2-year PFS of 44 %) [90]. A recent phase II study for newly diagnosed elderly patients with MCL treated with bortezomib in combination with doxorubicin, dexamethasone, chlorambucil and rituximab (RiPAD + C regimen) showed a ORR of 79 % with 51 % CR and a median PFS of 26 months. The scheme was toxic, however, with 2 treatment-related deaths (5 %), 4 patients (10 %) discontinuing the treatment because of severe toxicity and 7 patients (18 %) experienced grade 3 neurotoxicity [91]. Moreover, two clinical trials are currently ongoing, assessing the combination of BR plus bortezomib or lenalidomide in first-line treatment of MCL patients (NCT01415752 and NCT00963534, respectively, Table 9.3).
First-Line Treatment for Elderly Frail Patients
If treatment of elderly frail patients is considered, this should consist of mild immunochemotherapy, as chlorambucil combined with rituximab, which is usually very well tolerated [92, 65]. All treatments should be given with the perspective that cure will not be obtained, and that palliation should aim at improvement of quality of life [34].
Bendamustine is also an active monotherapy that is well tolerated in older or frail patients and may be discussed in combination with rituximab in selected cases of this patients subset [80–82].
Single agent therapy with rituximab (four gifts at weekly intervals) for treatment-naive patients is not recommended, as only very low ORR of 27 % with 3 % CR have been obtained. Continuation of maintenance therapy with rituximab did not further contribute and caused up to 13 % grade 3–4 hematologic toxicity [93].
Consolidation and Maintenance Therapy
Although high response rates have been achieved by the discussed therapeutic approaches, survival curves do not show any plateau, as almost all patients will finally relapse after induction treatment and most likely die of recurrent disease. Thus it is crucial to implement additional consolidation concepts (e.g. rituximab maintenance, radioimmunotherapy –RIT- consolidation, new molecules within studies) to maintain long lasting remissions.
In the past, maintenance therapy with interferon-alpha has been applied in several studies, but the beneficial effects on PFS did not reach the statistical significance [7, 94].
In the abovementioned European MCL Network Elderly trial, responding patients were randomized between a maintenance phase with interferon alpha or rituximab. In fact, rituximab maintenance reduced the risk of progression or death by 45 % (58 % patients in remission after 4 years vs. 29 % with interferon alpha), almost doubled duration of remission and significantly improved OS among patients responsive to R-CHOP [79]. Thus, rituximab maintenance (1 dose every 2 months until progression) should be offered to all patients responding upon R-chemotherapy, especially R-CHOP induction [34].
In addition to rituximab, various other candidates might be suitable for maintenance therapy. Lenalidomide seems attractive, either alone or combined with rituximab, and is currently being tested in the Italian randomized trial FIL-MCL0208 for young patients after auto-SCT (EudraCT Number 2009-012807-25). A comparison in older patients of additional lenalidomide with rituximab maintenance versus rituximab alone is being investigated in the current European MCL Network ‘‘MCL R2 Elderly’’ randomized trial (EudraCT Number 2012-002542-20, Table 9.3).
Despite some concerns about its cumulative neurotoxicity, bortezomib maintenance is currently also being tested in the Dutch-Belgian HOVON 75 trial for young patients after high dose induction therapy and auto-SCT (EudraCT Number 2006-000386-11).
Finally, promising data have been achieved by RIT consolidation in elderly patients, too. Four cycles of R-CHOP followed by yttrium-90 (90Y)-ibritumomab tiuxetan (an anti-CD20 radio-immunoconjugate antibody) compared favourably with historical results of six cycles of R-CHOP in patients with previously untreated MCL. This regimen was well tolerated and may be applicable to most patients [95].
A list of the main ongoing clinical trials for elderly MCL patients is shown in Table 9.3.
Treatment of Limited Stage Disease
Similarly to other non-Hodgkin lymphomas, one might consider to treat localized disease (stage I/II) with limited immunochemotherapy followed by involved field radiotherapy [65]. Stage I disease is, however, rare in MCL, with the large majority of patients presenting with advanced stage IV disease and usually bone marrow involvement. Moreover, no reliable data of randomized clinical trials are available. Two retrospective Canadian analyses, each accounting for 26 patients with limited disease treated with various combinations of radiotherapy and/or chemotherapy, suggested an important role for radiotherapy [66, 96]. Radiotherapy alone by applying involved field or even extended field radiotherapy might be considered for frail elderly patients with limited stage I/II disease [68], but achieved only temporary remissions in an ongoing trial of the German Lymphoma Study Group (Martin Dreyling, personal communication 2013).
Treatment of Relapsed Disease
Almost all patients will finally relapse after induction therapy. In younger patients relapsed lymphoma may have a curative approach based on allogeneic transplantation [97, 98]. Given the difficulties to offer elderly patients dose-intensified salvage therapy with or without stem cell transplantation (autologous or allogeneic), the therapeutic goal of relapsed MCL should be the prolonged disease control. For selection of optimal treatment, efficacy versus toxicity, but also the ease of administration (for example orally versus intravenously) have to be balanced and discussed with the patient. In addition, new treatment modalities should be offered in the context of clinical trials, especially to the elderly patients, as they have a very poor outcome once relapsed [68]. Therefore (if not in a palliative situation) these patients should be generally referred to experienced centers to determine the optimal therapeutic strategy. A list of the major current clinical trials for elderly MCL patients is provided in Table 9.3. In general, treatment strategies should depend on the individual risk profile and patients fitness: hence a CGA should be always performed to define the best treatment option for relapsed patients, too. In this regard the most recent data of salvage regimens are discussed below (Table 9.2).
Immunochemotherapy Combinations
Most elderly patients received R-CHOP as first line treatment. A well established salvage regimen, based on purine analogues, is R-FC or R-FCM [78]. Conversely, if a purine analogue was included in the first line treatment, an anthracycline based regimen at relapse may be considered (R-CHOP) [68]. However, although the addition of rituximab to conventional chemotherapy (R-FCM, R-GEMOX, R-DHAP) increased response rates up to 60–70 %, the duration of response in relapsing disease remains limited (mainly less than 1 year) and such treatment options should be considered basically palliative [78, 99, 100].
As already discussed before, more promising data have been recently achieved by the combined regimens with bendamustine, such as the well tolerated BR and the even more effective but also more toxic R-BAC regimen [80, 84]. Thus, depending on patients performance status and comorbidities, a treatment comprising bendamustine should be preferred in relapse setting for elderly patients.
Finally, for a palliative approach, the efficacy, feasibility and low toxicity of an oral low-dose metronomic polichemotherapy combination (PEP-C) is noteworthy and such well tolerable regimens should be always considered for elderly patients, optionally in combination with rituximab and thalidomide [101, 102].
Established Molecular Targeted Approaches
The first “new drug” to be registered in relapsed MCL in US has been bortezomib, based on its selective and reversible proteasome 26S inhibitor efficacy [85–87]. Although the combination of bortezomib with rituximab and chemotherapy showed high response rates (up to 60–70 %), median PFS rates among heavily pre-treated patients were only in the range of 12 months. The published regimens encompass both combinations with rituximab and steroids [103, 104], as well as combinations with immunochemotherapies such as rituximab, dexamethasone and high dose-cytarabin (R-HAD), rituximab, prednisone and cyclophosphamide (R-CP), rituximab-bendamustine (BR) and gemcitabine [105–108]. The toxicity profile predominantly consists of polyneuropathy and neutro-thrombocytopenia. A phase III clinical trial is currently ongoing, randomizing MCL relapsed patients to receive either R-HAD ± bortezomib (NCT01449344).
Temsirolimus, an intravenous mammalian target of rapamycin (mTOR) inhibitor, received the European Medicines Agency approval in 2009, due to its single-agent activity in patients with relapsed MCL. Temsirolimus induced a significant improvement in median PFS and ORR, compared with investigator’s choice monotherapy (4.8 vs. 1.9 months and 22 % vs 2 %, respectively) [109]. Hematological adverse events were generally well managed by dose reductions or treatment delay. Temsirolimus should be considered in advanced relapses (greater than second line), especially for non fit patients [34]. The addition of rituximab to temsirolimus was subsequently tested in a phase II study on 71 patients. An increased ORR of 59 %, with up to 19 % CR was observed, with a median time to progression (TTP) of about 10 months [110]; the toxicity profile was similar to temsirolimus monotherapy. To further improve its efficacy, temsirolimus is being currently investigated in combination with BR in a phase II clinical trial (NCT01078142) [111].
Another well tolerated oral mTOR inhibitor is Everolimus [112], but further studies in combination with chemotherapy or other biological drugs are warranted.
A number of phase II trials have confirmed the promising response rates of the immunomodulatory compound lenalidomide in relapsed MCL [88, 89, 113]. Recently a phase II study in 52 patients with relapsed MCL confirmed the impact of a chemo-free lenalidomide-rituximab combination with high response rates (57 % ORR, 36 % CR) and impressive response durations up to 19 months [114]. The manageable toxicity (mainly mild hematological) and the oral formulation make this drug an attractive option also in the context of maintenance regimens. A phase II trial of the rituximab-lenalidomide-bendamustine combination + lenalidomide maintenance is ongoing for relapsed MCL (NCT01737177).
Largely overshadowed by its subsequent follower lenalidomide, thalidomide is an active compound in relapsed MCL, with a favourable side effect profile (7 % grade 3–4 adverse events, including thromboembolism), with an interesting ORR up to 50 % (plus 29 % SD, stable disease) and a time to treatment failure (TTF) of 29 % and 11 % at 1 and 2 years, respectively [115, 116]. Thus, thalidomide might offer a cost-effective alternative to more expensive targeted agents, especially in countries with limited health-care resources [117].
In contrast to its favorable safety profile (mainly manageable thrombocytopenia and neutropenia) and promising data in other types of lymphoma, monotherapy RIT with 90Y-ibritumomab tiuxetan does not impact substantially the prognosis of relapsed MCL, with an ORR of 32 % and a EFS (event free survival) of 6 months [118]. Thus recent studies are exploring its combination with others efficient targeted drug, such as bortezomib. [119]
Innovative Molecular Targeted Approaches
The growing insights into the underlying molecular biology of MCL form the basis for the ongoing exploration of targeted approaches [21]. A number of new compounds are currently being tested in MCL and are available for the application within clinical trials.
The most convincing data come for the oral Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib that specifically blocks the B-cell receptor (BCR) signaling survival pathway. In an international phase II trial on refractory/relapsed MCL patients ibrutinib monotherapy showed impressive efficacy and excellent tolerability with an impressive ORR of 68 %, 22 % CR and a PFS of around 14 months. Noteworthy is the favorable safety profile, with less than 15 % grade 3/4 hematological toxicity and mainly mild gastro-intestinal symptoms, fatigue and infections in a population of heavily pre-treated patients [120]. Two phase III trials are currently ongoing, the first comparing ibrutinib versus temsirolimus in relapsed patients (NCT01646021) and the second investigating a BR schedule ± ibrutinib as first line treatment (NCT01776840).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree