(1)
Daytona Beach Shores, FL, USA
Insanity: doing the same thing over and over again and expecting different results.
– Albert Einstein
What has the cell-kill paradigm and its dominance of cancer research, diagnosis, treatment, and outcome assessment achieved in the context of the “War on Cancer” since the enactment of the National Cancer Act of 1971? The answer will vary depending on how achievement is measured and who does the assessment. For example, in a 1996 review article titled The war on cancer [505] marking the 25th birthday of the National Cancer Act of 1971, the author used a quote from Charles Dickens to dramatize its failure: “Dead, your Majesty. Dead, my lords and gentlemen. Dead, right reverends and wrong reverends of every order. Dead, men and women, born with Heavenly compassion in your hearts. And dying thus around us every day”. Less than a year later, an editorial written by a former Director of the NCI rejoiced “Happy birthday ‘War’, you deserve a pat on the back” [506]. Both authors converged on crediting major scientific advances made during this period, especially the breathtaking advances in molecular biology and molecular genetics, including the genome project, that have revolutionized our knowledge about cancer. Yet, while both see a brighter future after these advances are applied to the practice of medicine, the former author concluded, “We must develop new approaches to control this plague of deaths, adopting an ethic of prevention ….to prevent disease before it becomes invasive and metastatic” [507].
Such drastically contrasting perceptions of the War on Cancer achievements seem surprising, for a dispassionate analysis of the facts should provide a clear and objective answer. However, selection and interpretation of some statistical endpoints can support different points of view. For instance, in a recent article, ACS staffers examined “trends in death rates for all cancers combined and 19 common cancers from 1970 to 2006 and review the contribution of prevention, early detection, and treatment to reducing cancer death rates”, concluding,
While such a conclusion places prevention, early detection, and treatment on an equal footing, the authors correctly noted, “Lung, female breast, prostate, [and] colorectum [cancer] accounted for about 60–80 % of the total decrease in all-cancer death rates since 1990/91”, which they attributed, also correctly, to smoking cessation and to screening for surgically resectable breast, colorectal, and prostate cancer. This implies a rather modest contribution by advanced cancer treatment to patient survival, despite the rhetoric and high cost of new cancer drugs in recent years, especially with the introduction of targeted therapeutics. For instance, the monthly Medicare price for Proleukin® was $13,503 at approval time (1992), $19,925 for Campath® (2001), and $19,425 for Arranon® (2005). Moreover, the high initial cost of some agents has increased over time, as exemplified by Gleevec® that cost Medicare $3,401 per month in 2001 but more than doubled to $92,000 annually to become a blockbuster drug for its manufacturer Novartis, with sales of $4.7 billion in 2012 [509]. The price escalation of 101 cancer drugs between the 1970s and 2008 has been compiled recently, comparing, on an interactive graph, monthly prices on approval to those in 2007 [510]. In a recent joint communication, 100 CML experts lamented, “Of the 12 drugs approved by the FDA for various cancer indications in 2012, 11 were priced above $100,000 per year” [511]. In addition to blockbuster drugs, “orphan” drugs1 can be equally profitable, not because of their wide distribution but because of their unit price. For instance, Kalydeco®, a drug for first cystic fibrosis, costs $290,000 annually, while at $410,000 per patient per year, Soliris®, a drug used to treat paroxysmal nocturnal hemoglobinuria, is the world’s priciest. It is to be noted that the essential “carte blanche” that pharmaceutical companies enjoy in pricing the new crop of targeted drugs will escalate cancer treatment costs so that only the well-off or the well-insured can afford them, marginalizing large populations in both rich and poor countries.
Progress in reducing cancer death rates is evident whether measured against baseline rates in 1970 or in 1990. Downturns in overall cancer death rates since the early 1990s are largely a result of tobacco control efforts beginning in the 1960s, screening and early detection for several cancers disseminated in the 1980s and 1990s, and modest to large improvements in treatment and survival for specific cancers [508].
Other cancer experts, after years of clinical and research experience and a detailed multifactorial analysis of the facts, have pointed out that decreased cancer mortality reflects lower incidence and increased early detection with minor improvements in cancer treatment [512], and that,
Factors other than treatment have contributed to lower mortality rates after 1992, and to increased survival over several decades. While the latter is due mostly to improvements in overall health care over time, the former resulted from public education campaigns that foster prevention via reduction in environmental and behavioral risk exposure, and early stage diagnosis via screening programs. Overall, fifty years of cytotoxic chemotherapy contributed minimally to the modest improvements in mortality rates or survival. This is because the faulty cell-kill paradigm, that views cancer as a “new growth” distinct from the host that must be eradicated at any cost, has misguided drug development and patient care for decades [513].
More recently (October 2012), the status of the war against cancer was reviewed by leading epidemiologists, researchers, clinicians, policy makers, cancer advocates, and industry representatives, who gathered in Lugano, Switzerland at the World Oncology Forum (WOF). Their consensus conclusion was even harsher:
At the final session, participants issued an appeal to world leaders to fulfill commitments made at the World Health Assembly in May 2012 to cut preventable deaths from non-communicable diseases by 25 % by 2025. The Stop Cancer Now! appeal was published in major newspapers on World Cancer Day (4 February 2013) followed by a subsequent editorial in The Lancet [515]. WOF participants’ broad-based, if somewhat diffuse, 10-point plan rests mainly on waging a worldwide war against tobacco, developing early detection of cancers that are the most detectable, treatable, and have the greatest social impact, providing optimal pain control by removing bureaucratic, legal, and logistical barriers to the medical use of morphine, and accelerating delivery of affordable therapies to benefit patients across the world [516].
Current strategies for controlling cancer are clearly not working: preventable cancers are not being prevented; patients are suffering and dying unnecessarily from cancers that are detectable and treatable; and the model for developing effective new curative therapies is not fit for purpose and needs a radical rethink [514].
It is clear that in order to make progress in the War on Cancer, we must identify where progress has been made and where it has not, how progress is measured, and what confounding factors might impact data analysis. Only then will we be in a position to advocate and implement changes necessary to impact cancer patients’ lives, to which the concept of value is central. Yet, while perception of what constitutes value in cancer care varies among stakeholders, patients’ perception must predominate over all others, as pointed out at a recent conference convened by the drug industry,
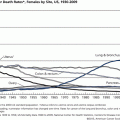
In that context, most patients agree that disease prevention, survival, and QOL epitomize the most meaningful issues they care about. Yet, interpretation of the latter two must take into account a host of impacting factors that, while tangential, are decisive for guiding and formulating cancer policy. Some of the most obvious confounding factors include recent shifts towards early-stage and slow-growing tumors and overall improvements in general medical care over time. For example, increasing numbers of early-stage and slow-growing tumors fostered by better screening tools contributed to rising incidence and survival rates, at least temporarily, while new screening tests are being implemented nationwide, as in the case of breast and prostate cancer. Likewise, refinements in cancer staging techniques have contributed to “stage migration” over time. That is, patients with occult metastases undetected in the era preceding CAT scans and MRI were classified as having local or regional disease, whereas they are now included in the advanced stage category. As a result of their removal from the former group and their inclusion in the latter, the average survival for both groups has risen. This is because fewer poor prognosis patients are included in the former group and the latter now includes patients with “early” advanced disease (based on CT or MRI staging), whereas in the past it was populated by patients with clinically far-advanced disease. An obvious contribution to declining incidence and death rates is linked to prevention and early-stage detection. The classic example is lung cancer where an approximately 30 % decline in lung cancer mortality after reaching a peak in 1991 is due to a 1.1 % decline in annual incidence rates linked to decreasing smoker populations rather than to early diagnosis or improved treatment. On the other hand, the average 2.1 % annual decline in breast cancer mortality rates between 2000 and 2009 was caused by increasing percentages of cases diagnosed in operable and potentially curable early stages [518], and to a lesser degree, to improvements in the management of intermediate stages. Less obvious is the impact of improvements in general medical support measures, such as potent antibiotics for treating chemotherapy-associated infections, easier access to blood product transfusions, and other life-sustaining measures that contribute to survival of previously fatal treatment complications. Efforts to control cancer necessarily must rest on three stools: prevention, early-stage diagnosis through screening, and treatment of patients who, for one reason or another, exhibit advanced-stage cancer at diagnosis. Hence, benchmarks to monitor must include trends in incidence rates to assess the effectiveness of preventive measures, survival and mortality rates to assess screening and treatment efficacy, and QOL to judge the impact of all cancer management efforts combined, but most particularly treatment of advanced-stage cancer [519].
…value is ‘in the eye of the beholder’ and to which perceptions of value (both clinical and economic) vary among stakeholders, including patients, industry, insurers, politicians, physicians, advocacy and interest groups…the patient’s concept of value must be given a primary role at the center of the cancer care ecosystem [517].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree