Fig. 2.1
Estimated annual numbers of transplants in the USA were compiled according to the number registered with CIBMTR. Estimates of how closely the numbers reported are representative of actual transplant activity vary according to the type of transplant and number of centers reporting data per year. Prior to 2007, all except unrelated donor allogeneic transplant facilitated by the NMDP were reported voluntarily. It was estimated that the CIBMTR captured 90 % of all unrelated donor transplants performed in the USA, 60–90 % of related donor allogeneic transplants and 65–75 % of autologous transplants. These estimates were extrapolated from other databases that capture transplant center activity, accreditation, or hospital discharges. After 2007, the Stem Cell Transplant Outcomes Database (SCTOD) was initiated which changed reporting requirements and data capture to an electronic format. The SCTOD requires that all allogeneic transplants performed in the USA be registered with CIBMTR. Data reporting of autologous transplants remains voluntary and the numbers in the CIBMTR database are estimated to be 80 %. US numbers of allogeneic transplants in the CIBMTR are representative of the actual transplant activity. The number of autologous transplants in the USA has steadily increased since 2000, mainly for treatment of plasma cell and lymphoproliferative disorders. The ongoing increase of autologous transplants is likely related to a higher number of patients older than 60 years being performed nationwide. Allogeneic transplants from unrelated donors surpassed the number of allogeneic transplants from related donors after 2006 and the gap between these two types of approaches continues to widen annually. The major contributing factors to this trend are the growth of unrelated donor databases, improvements in unrelated donor transplant, and increase in numbers of allogeneic transplants for patient older than 60 years with reduced intensity conditioning. (Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides, 2013. Available at: http://www.cibmtr.org)
HSCT has demonstrated efficacy for the treatment of selected malignancies (e.g., multiple myeloma, acute and chronic leukemia, lymphoma) , as well as for immunodeficiency, bone marrow failure, and infiltrative disorders such as amyloidosis. The development of reduced intensity-conditioning regimens has allowed successful treatment of older patients and those with comorbidities that would deem them ineligible for myeloablative therapy (see Fig. 2.2).
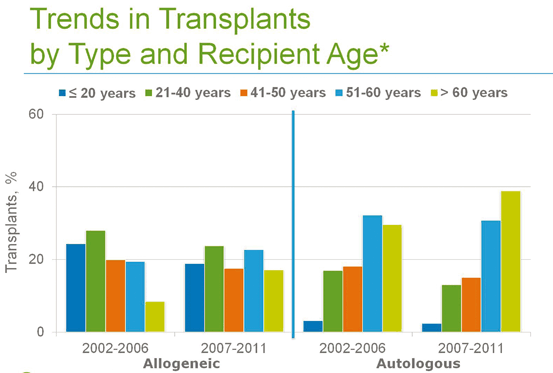

Fig. 2.2
The number of autologous and allogeneic transplants for treatment of malignant diseases in older patients continue to increase. Thirty-nine percent of autologous transplant recipients and 17 % of allogeneic transplant recipients in 2007–2011 were older than 60. The majority of autologous transplant recipients (70 %) and 40 % of allogeneic transplant recipient were older than 50 in this later period. Among allogeneic transplant recipients, the proportion of patients older than 60 years doubled from 8 % to 17 % during the decade analyzed. (Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides, 2013. Available at: http://www.cibmtr.org)
Finally, the expansion beyond human leukocyte antigen (HLA)-identical sibling allogeneic HSCT to unrelated donor transplants as well as alternative donors, including unrelated cord blood transplants and related haploidentical donors , has resulted in donor availability for nearly all patients in need.
2.1 Increase in Utilization and Impact of HSCT on National Health-Care Costs
The amplification in numbers of HSCT procedures has been associated with a dramatic increase in overall costs. Utilization of unrelated cord blood products has further impacted expenditure, as those patients generally experience slower hematopoietic and immunologic recovery, requiring increased resource utilization.
The improved survivorship of cancer patients has been confirmed as recently reported by the National Cancer Institute (NCI). Annual expenditures on cancer have also increased in the USA with cancer-care costs estimated at US $ 124.6 billion in 2010, of which, the transplantable malignancy of lymphoma was #3 and leukemia was #6 in expenditure by disease sites. Costs are estimated to exceed US $ 160 billion by 2020. The increase in HSCT utilization was substantiated in a recent report from the Agency for Healthcare Research and Quality (AHRQ) of an analysis performed by the Healthcare Cost and Utilization Project (HCUP) of the Nationwide Inpatient Sample, a database of hospitalization and inpatient stays, representative of all short-term, nonfederal hospitals. For activity between January 2004 and December 2007, it was shown that the HSCT procedure was ranked highest in percentage increase for commonly performed inpatient procedures for hospital costs (84.9 %) and for total hospital stays (51.3 %) with approximate costs of US $ 1.28 billion in 2007 (Table 2.1). Recognizing that the HSCT procedure represented approximately 1 % of total hospital stays, 4.4 % of the total costs were encumbered for HSCT.
Table 2.1
AHRQ analysis of medical and surgical procedures with increased utilization in the USA. Commonly performed procedures with the most rapidly increasing hospital inpatient costs, 2004–2007. (AHRQ, Center for Delivery, Organization, and Markets, Healthcare Cost and Utilization Project, Nationwide Inpatient Sample, 2004 and 2007)
Principal procedure category | Total costs (2007) (US $) | Total hospital stays (2007) | Percentage change | |
|---|---|---|---|---|
Total costs (2004–2007) (%) | Total hospital stays (2004–2007) (%) | |||
Bone marrow transplant | 1,282,645,000 | 15,100 | 84.9 | 51.3 |
Open prostatectomy | 1,032,016,000 | 88,500 | 68.6 | 40.8 |
Aortic resection; replacement or anastomosis | 1,872,908,000 | 61,600 | 38.5 | 31.9 |
Cancer chemotherapy | 2,616,504,000 | 187,400 | 33.2 | 14.2 |
Spinal fusion | 8,863,922,000 | 350,700 | 29.5 | 15.6 |
Lobectomy or pneumonectomy | 1,757,748,000 | 81,400 | 29.2 | 24.9 |
Incision and drainage, skin and subcutaneous tissue | 1,108,187,000 | 158,600 | 28.6 | 31.5 |
Arthroplasty knee | 9,217,740,000 | 605,200 | 27.5 | 25.7 |
Nephrotomy and nephrostomy | 682,609,000 | 38,600 | 25.3 | 11.7 |
Mastectomy | 660,173,000 | 70,100 | 23.8 | 3.6 |
Total for top 10 procedures a | 29,094,452,000 | 1,657,100 | 32.3 | 22.2 |
This rapid increase in HSCT procedures took place in a 48-month interval within the past decade. However, these numbers are a small fraction of what is currently projected for the near future. Based on population demographics and surveillance, epidemiology, and end results (SEER) data for the incidence and prevalence of malignancies, the National Marrow Donor Program (NMDP) anticipates a doubling of the current number of unrelated transplants performed (~ 5500 in 2011) as early as 2015 (estimated as high as 12,500 procedures). They also predict a concomitant 30 % increase in autologous HSCT.
These reports from the HCUP and the NMDP are supported by the Milliman 2011 US Organ and Tissue Transplant Cost Estimates and Discussion report. The analysis suggests that there was a 110 % increase in billed charges for allogeneic HSCT between 2003 and 2008. The estimates were based on billed charges (recognizing that charges do not equate to cost of procedures nor do charges indicate what percent of charges are paid by the governmental or private payer). Autologous transplant charges increased from approximately US $ 205,000 to US $ 370,000, and allogeneic transplant charges increased from approximately US $ 380,000 to US $ 805,000 in this short period of time. Also, recognizing that approximately 20,000 procedures were performed, these individual numbers suggest that transplantation may become a US $ 10 billion industry.
2.2 Complexity of Care Increases Costs
In the setting of increasing demand for HSCT and increasing cost of health care and novel technologies, it remains critical for providers and health systems to assure that adequate reimbursement is obtained to cover the costs of the individual procedures, costs associated with the defined incident of care, and the potential associated with medical complications and sequelae.
Reimbursement based on a fee-for-service indemnity approach no longer exists for the vast majority of patients. Insurance carriers have developed case rate contacts for HSCT with negotiated payments for pretransplant evaluation, HLA typing, transplant product acquisition, and patient care. In contrast, government payers (Medicaid and Medicare) have set reimbursement schedules:
1.
Medicare coverage provides funding for a period of time surrounding the actual transplant procedure, typically in a diagnosis-related group (DRG)-based reimbursement structure.
2.
It is important to recognize that DRG payments are provided with the presumption of a predictable resource consumption encountered by the recipient.
3.
In some instances, the payer does not differentiate between autologous , allogeneic-related, and allogeneic-unrelated transplant in their rate-setting process:
a.
This approach ignores the greater complexity of workup, cell source selection, and post-treatment risk of complications for the allogeneic recipient.
4.
Preexisting comorbidities as well as the disease state and donor type drive resource consumption. These variables, seen across the spectrum of patients for whom transplant services are provided, are not accounted for by the limited DRG codes.
Contractual arrangements with private/commercial payers will often carve out HSCT services from general medical services contracts:
1.
Services related to HSCT will often have a bundled payment for all services performed within a boundary of time around the transplant, usually covering the first 30 days for an autologous and 100 days for an allogeneic HCST procedure.
2.
These contracts should be designed to cover:
a.
Recipient evaluation and assessment of transplant eligibility
b.
Donor search benefits
c.
Harvest and acquisition of stem cell product
d.
The immediate peri-transplant period and the post-transplant phase
e.
Special circumstances (preplanned second transplant procedure, donor leukocyte infusion, retransplants, high-cost pharmaceuticals (e.g., plerixafor)
2.3 Contracts and Reimbursement Strategies
If structured appropriately, contracts should reflect mutual exposure to financial risk. Reimbursement methodologies vary in the degree to which financial risk is shared.
One of the confounding issues that those involved in the care of the transplant patient face is that the actual transplant procedure is generally an infusion that occurs at a precise moment in the midst of a complicated medical treatment course. The infusion defines the actual transplant. However, reimbursement usually is focused on providing coverage for that event and for a series of surrounding days, which defines an episode of care. Various reimbursement methodologies have been undertaken, including reimbursement of:
1.
All charges generated by providers and health systems in care of an HSCT patient
2.
A discount of charges which actually represents a fixed rate percent, discounting total billed charges
3.
A case rate, which incorporates a fixed fee that covers all transplant-related hospital or clinic services for a specified period of time, predating and following the actual infusion event
4.
A global case rate which represents a fixed fee that covers all hospital and physician charges for a specified period of time, typically involving post-transplant care
Recognizing the unique needs of individual patients, many of the case rate and global case rate methodologies will include provisions that protect the transplant center as well as the payer from financial risk. These provisions vary in the degree of financial protection they provide. Examples include:
1.
Outlier days, which provide a per diem reimbursement for each inpatient day beyond a well-defined post-infusion time period
2.
An outlier threshold which reimburses the provider and institutions a defined percentage of billed charges after a specified threshold beyond the case rate has been reached
3.
A floor provision which assures that at no time will a hospital be reimbursed less than a specific percent of billed charges
The setting in which the HSCT procedure is performed, i.e., inpatient or outpatient, may influence reimbursement . Pharmaceuticals may be reimbursed at a higher level per dollar of charge in the outpatient setting. The differences in reimbursement based on setting can have a significant impact on the financial performance of the HSCT program.
2.4 Integrated Structure for Contract Management
The complexity of contracting for HSCT services is reinforced by the implementation of separate transplant specialty contracting personnel by hospitals and payers. Development of rate structures that support the center’s strategic initiatives, monitoring of the center’s performance on each contract, and providing assistance to patients in understanding their benefits as they relate to the contract require an integrated team approach:
1.
A typical team for contract management would include:
a.
Managed care contracting
b.
HCST program medical director
c.
HSCT program administrator
d.
Patient billing services
e.
Financial counseling personnel
f.
Program’s managed care clinical liaison/coordinator:
i.
Review of patient referral insurance information
ii.
Review of patients’ benefits:
Lifetime maximum
Transplant maximum
Prescription coverage
iii.
Communication with patient regarding benefits
iv.
Liaison with insurance company in communication of patients’ status in the process
g.
Medical social worker
2.5 Payer Types
Understanding reimbursement variability between governmental and private payers is a necessity. Traditionally, since HSCT was performed in younger patients, private payers dominated the health coverage. However, over the last half decade, there has been a significant change in the payer mix with an increase in patients with governmental insurance support (Medicare or Medicaid) .
According to transplant center estimates, as many as 25–30 % of their patients were supported by governmental payers in 2012, an increase from previous estimates of approximately 15 % in 2007. This shift in payer mix can have a dramatic impact on transplant program financial viability, given the low average rates of reimbursement by Medicare and state Medicaid programs.
1.
Get Clinical Tree app for offline access

Affordable Care Act:
a.




The Patient Protection and Affordable Care Act (ACA) was signed into law on March 23, 2010 and could add more than 30 million Americans to the insured ranks by 2019.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



