Thalidomide and Lenalidomide
Pearl O. H. Ugwu-Dike
Yevgeniy Semenov
Thalidomide and lenalidomide are antiangiogenic, antineoplastic, immunomodulatory agents used in the management of a wide range of conditions. Thalidomide, historically used as an antiemetic and sedative for pregnant women, gained notoriety for its associated risk of teratogenicity.1 Current U.S. Food and Drug Administration (FDA)-labeled indications for thalidomide include multiple myeloma and cutaneous manifestations of erythema nodosum leprosum.2 Off-label indications for thalidomide use in adults include acquired immunodeficiency syndrome-related aphthous stomatitis, chronic graft-versushost disease, systemic light chain amyloidosis, and Waldenström macroglobulinemia.2 Lenalidomide, a thalidomide analog, has similar FDA-labeled indications for hematologic malignancies: multiple myeloma, follicular lymphoma, mantle cell lymphoma, marginal zone lymphoma, and myelodysplastic syndromes.3 Off-label indications for lenalidomide use in adults include chronic lymphocytic leukemia, diffuse large B-cell lymphoma, smoldering myeloma, and systemic light chain amyloidosis.3
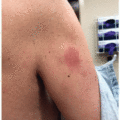
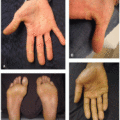
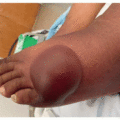
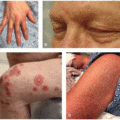
While thalidomide and lenalidomide show increasing promise as valuable agents in cancer and inflammatory disease therapy, both medications carry the potential for significant adverse drug reactions. In addition to rash, which affects 40% to 50% of patients, common side effects of thalidomide include sedation, headache, peripheral neuropathy, constipation, malaise, pain, vertigo, impotence, and thrombosis.2,3 Thalidomide-induced cutaneous toxicity is typically seen 10 to 14 days after treatment initiation, although rashes have been reported up to 4 months after treatment start. The spectrum of skin toxicities associated with thalidomide varies, and the most frequently documented morphologies include morbilliform eruptions, vesicular presentations, erythema with pruritus, or seborrheic eruptions.3 More severe adverse reactions such as toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), and exfoliative erythroderma can also occur.4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree