(1)
Institut Bergonié, Bordeaux, France
Abstract
Transforming growth factor beta (TGFβ) is the lead of a large family of factors studied in this chapter, which comprise several stricto sensu TGFβs and a subfamily of related factors, and a subfamily of bone morphogenetic factors (BMPs). The receptors of these factors display serine/threonine kinase activity, which distinguishes them from those presenting (or are associated to) tyrosine kinase activity (Chaps. 1 and 4). They are involved in many cellular processes, especially during development, and play a role in cell survival, proliferation and differentiation, as well as in cell adhesion and motility, and its alterations are found in cancer, inflammation and fibrosis. Overall, the TGFβ pathway generally works in opposition to cell proliferation and plays a tumour suppressor role counteracting oncogenesis, but the role of this pathway is ambivalent since it frequently enhances cell motility and migration, participates to the epithelial-to-mesenchymal transition and, consequently, may favour metastasis dissemination.
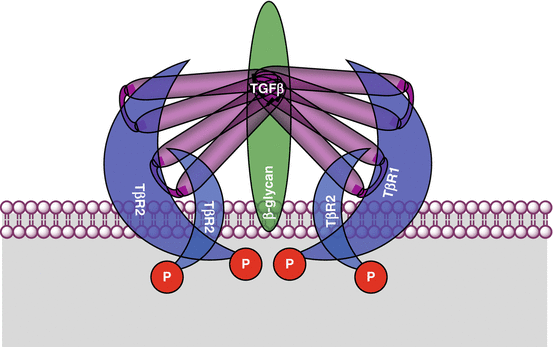
The factors of the TGFβ family are active as homodimers and are responsible of the formation, at the level of the target cell, of a hexameric complex made of two molecules of ligand, two molecules of type I receptors and two molecules of type II receptors, in addition to coreceptors required for signalling. Once activated by ligand binding, type II receptors phosphorylate type I receptors, which in turn phosphorylate transcription factors of the SMAD family. This denomination comes from the names of the ortholog genes sma (small) of Caenorhabditis elegans and mad (mothers against decapentaplegic homologue) of drosophila. The SMAD factors bind to a common interactant, also of the SMAD family, and form a transcriptional activator or repressor complex which is able to relocate to the nucleus and regulate the transcription of target genes, which are more or less specific of the extracellular factor generating the signal.
5.1 TGFβ Family Ligands
Table 5.1 inventories the main factors of the lato sensu TGFβ family, whose total number exceeds 30. These factors and their receptors may have very variable tissue distribution and are expressed at definite periods of life: some of them are expressed only in some cell types and/or during a very short period of embryonic life, while others are ubiquitous and/or expressed all life long. In the TGFβ subfamily are found three TGFβs stricto sensu; a series of proteins called inhibins or activins, nodal, lefty and myostatin; as well as GDF (growth differentiation factors) 1 and 3. In the BMP subfamily are found BMP 2, 4, 5, 6, 7, 9 and 10; GDF 5, 6 and 7; and AMH (anti-Mullerian hormone) also known as MIS (Mullerian inhibitory substance).
Table 5.1
Ligands of the TGFβ family (lato sensu) and their receptors
Ligand | Type I receptor | Type II receptor | Coreceptor | SMAD factor | |
|---|---|---|---|---|---|
TGFβ subfamily | TGFβ 1, 2, 3 | TβR1, ALK5 (TGFBR1) | TβR2 (TGFBR2) | β-glycan (TGFBR3) | SMAD2, SMAD3 |
TGFB1, 2, 3 | |||||
Activins A, B, C (INHBA, B, C) | ALK2 (ACVR1A) | ACVR2A | |||
ALK4 (ACVR1B) | ACVR2B | ||||
Myostatin (MSTN, GDF8) | ALK4 (ACVR1B) | ACVR2A | |||
ACVR2B | |||||
Nodal (NODAL) | ALK4 (ACVR1B) | ACVR2A | Cripto (CFC1, TDGF1) | ||
ALK7 (ACVR1C) | ACVR2B | ||||
GDF 1, 3 (GDF1, 3) | ALK4 (ACVR1B) | ACVR2A | Cripto (CFC1, TDGF1) | ||
ALK7 (ACVR1C) | ACVR2B | ||||
Inhibin (INHA) | ALK4 (ACVR1B) | ACVR2A | β-glycan (TGFBR3) | ||
ACVR2B | |||||
Lefty 1, 2 (LEFTY1, 2) | ALK4 (ACVR1B) | ACVR2A | |||
ALK7 (ACVR1C) | ACVR2B | ||||
BMP subfamily | BMP 2, 4 (BMP2, 4) | ALK3 (BMPR1A) | BMPR2 | RGMA | SMAD1, SMAD5, SMAD8 |
GDF 5, 6, 7 (GDF5, 6, 7) | ALK6 (BMPR1B) | ACVR2A | RGMB | ||
ACVR2B | |||||
BMP 6, 7 (BMP6, 7) | ALK2 (ACVR1A) | BMPR2 | RGMA | ||
ALK3 (BMPR1A) | ACVR2A | RGMB | |||
ALK6 (BMPR1B) | ACVR2B | ||||
BMP 9, 10 (BMP9, 10) | ALK1 (ACVRL1) | BMPR2 | Endoglin (ENG) | ||
Anti-Mullerian hormone (AMH) | ALK3 (BMPR1A) | AMHR2 | |||
ALK2 (ACVR1A) |
These factors are synthesised as precursor latent forms, which are later submitted to proteolytic cleavage releasing the active C-terminal part of the protein. The proteolytic enzymes in charge of this activation are convertases of the subtilisin family, such as furin, and collectively called PCSKs (proprotein convertases subtilisin/kexin) or PACEs (paired basic amino acid cleaving enzymes) (see Annex C). After cleavage, the three TGFβs remain non-covalently attached to the N-terminal peptide, called the latency-associated peptide, which is not the case for other factors of the TGFβ and BMP subfamilies. All TGFβs and BMPs are secreted as dimers and are characterised by the presence of a characteristic cysteine knot at the C-terminal level, which allows the formation of three intramolecular disulphide bridges; a last disulphide bridge is used for dimerisation (Fig. 5.1).
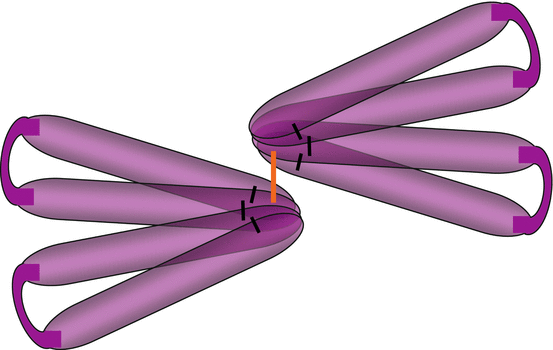

Fig. 5.1
Schematic representation of a TGFβ dimer. TGFβ structure reveals the presence of four ‘fingers’ linked by intrachain disulphide bridges (black lines) at the level of the ‘cysteine knot’. Two TGFβ molecules are bound by an interchain disulphide bridge (orange line)
All these factors are characterised by an antiparallel dimeric structure, which is formed before receptor interaction, allowing the recruitment of two molecules of type II receptors and of two molecules of type I receptor (Fig. 5.2). The ligands of the TGFβ subfamily have higher affinity for type II receptors, and this is only afterwards that two molecules of type I receptor are recruited and bound to the ligand-type II receptor complex. Some ligands of the BMP subfamily can bind indifferently to any receptor type, the complex thus formed recruiting afterwards the receptor of the other type. Dimerisation generally occurs between two identical molecules (homodimerisation); however, the dimerisation between an inhibin (INHA) and an activin (INHB) is possible, the heterodimer having a negative effect on receptor recruitment.