Fig. 19.1
The two stages of testicular descent. Between 8 and 15 weeks the gubernaculum or genito-inguinal ligament enlarges in male embryos under the action of Insl3 [augmented by MIS/AMH (?)]. This “swelling reaction” holds the testis near the groyne as the foetal abdomen enlarges, leading to transabdominal descent relative to the ovarian position. In the inguinoscrotal phase, the gubernaculum migrates out of the external inguinal ring, across the pubic bone and into the scrotum. The testis descends inside the processus vaginalis, which is a peritoneal diverticulum developing inside the gubernaculum. The inguinoscrotal phase is controlled by androgen
The inguinoscrotal phase of testicular descent is controlled by androgen [17, 18]. Intriguingly, there is a critical window for androgen effects on this phase, which in a rodent animal model is actually during the transabdominal phase of testicular descent. In foetal rats, the critical time for androgen effects on the second phase of descent is day 16–19 of development [19]. We have hypothesised that this is the time when the central nervous system is masculinised, so that the genitofemoral nerve can direct migration of the gubernaculum by release of calcitonin gene-related peptide (CGRP) [2] (Fig. 19.2). CGRP is known to have several effects on the gubernaculum in rodent models [20]. It was initially shown to cause rhythmic contractility of the cremaster muscle within the gubernaculum, which is suspected to be important for orientation of the direction of migration [21]. It also stimulates mitosis in the tip of the gubernaculum and inhibits apoptosis [22, 23]. However, it was unknown what caused the otherwise inert genito-inguinal ligament to undergo metamorphosis into an actively migrating structure.
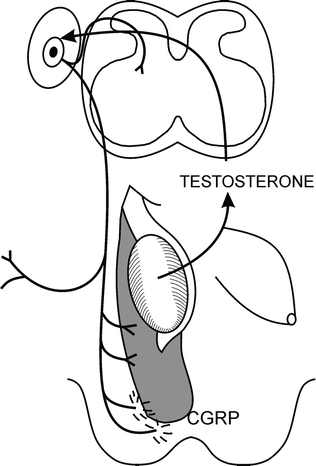

Fig. 19.2
The genitofemoral nerve hypothesis proposed that testosterone acted only indirectly on the gubernaculum by masculinising the genitofemoral nerve, which then produces a specific neurotransmitter (later identified as calcitonen gene-related peptide, CGRP) that is released from the nerve terminals to control gubernacular migration
We have shown recently that the gubernaculum acquires properties similar to an embryonic limb bud, with development of an active proliferating zone in its tip [24]. In an embryonic limb bud, the trigger for outgrowth comes from the overlying skin known as the apical epidermal ridge [25]. This specialised skin triggers the underlying mesenchyme to begin growing. We wondered, therefore, whether the inguinal skin provided similar signals to the gubernaculum [26]. With this in mind, it is interesting to note that the inguinal skin in marsupials has very special properties similar to a limb bud, as this is the site of the breast development in females and the scrotum in males [4, 27]. Importantly, the gubernaculum in marsupials is attached to the mammary gland in females and the scrotum in males [28]. The ilio-marsupialis muscle in marsupials is the female homologue of the cremaster muscle, and functions as a suspensory muscle of the nipples.
Relationship Between Gubernaculum and Mammary Bud
This intriguing relationship between the gubernaculum and the mammary gland in the marsupials stimulated us to look more closely at the embryonic rodent to see if there was a link between the gubernaculum and the mammary gland. When we examined the foetal rat and mouse, we found that there was an embryonic mammary bud immediately superficial to the gubernaculum in the inguinal region [29] (Fig. 19.3). In addition, the genitofemoral nerve supplied not only the gubernaculum but also mammary bud [30]. This raised the possibility that the mammary bud may supply signals to the underlying gubernaculum in a way similar to that seen in an embryonic limb bud. We have recently shown that there is expression of hoxa10 and fgf10 in the gubernaculum and the subcutaneous tissue forming the mammary fat pad [31].
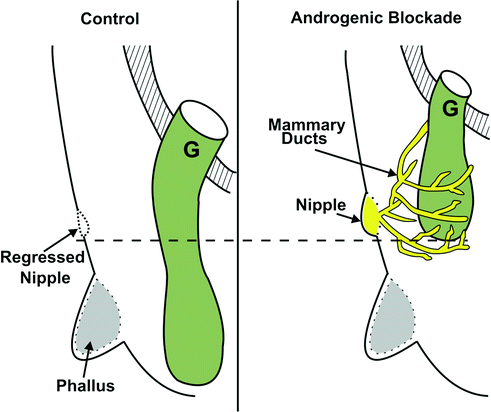

Fig. 19.3
Schema showing normal gubernacular migration from the external inguinal ring to the scrotum in rodents. Note the gubernaculum bypasses the regressing mammary bud and the phallus to reach the scrotum. After androgen blockade (with prenatal flutamide exposure), not only is gubernacular migration impaired, but also the mammary bud persists, and mammary ducts surround the gubernaculum [31]. (From Balic A, Nation T, Buraundi S, et al. Hidden in plain sight: the mammary line in males may be the missing link regulating inguinoscrotal testicular descent. J Pediatr Surg 45(2):414–8; discussion 418, with permission.)
At the time that we were investigating the role of the mammary gland and we were also trying to determine the site of androgen receptors that lead to masculinisation of the genitofemoral nerve. Initially, we had assumed that the receptors would be in the nerve itself [32]. As it is the sensory branches of the genitofemoral nerve that are most important for triggering gubernacular development, we looked for androgen receptors in the dorsal root ganglion. To our surprise, there are no androgen receptors in the dorsal root ganglion of the rat until after the critical window of androgen sensitivity on day 19 of foetal development [29]. In addition, there were no androgen receptors in the motor branches of the nerve at this time as well. When we looked at the gubernaculum, we were also surprised to find no androgen receptors until day 19 of development. However, the mammary buds and the adjacent mammary fat pad immediately outside the gubernaculum contained abundant androgen receptors during the critical window of androgen sensitivity [29]. Taking all these findings together, it is quite likely that androgens masculinise the genitofemoral nerve via the target organ, which in this case is the mammary fat pad. This would require the target organ to release a trophic factor that stimulates development of the nerves, which then produce calcitonin gene-related peptide, which is released from the sensory branches of the nerve to control migration of the gubernaculum (Fig. 19.4). There is some precedent for the possibility that target organs may stimulate androgen-sensitive development of the nerves, as the spinal nucleus of the bulbocavernosus is masculinised by a trophic effect from the muscle itself. In this case the trophic factor is brain-derived neurotrophic factor (BDNF) [33]. Whether a similar trophic factor is present in the mammary bud remains to be seen.
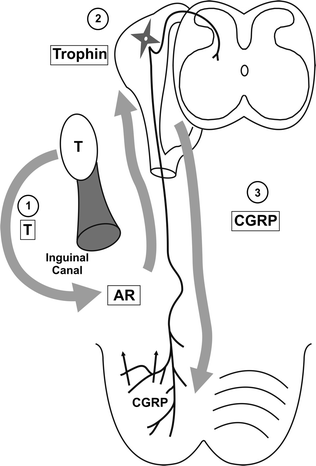

Fig. 19.4
Schema showing our current proposal for how androgens regulate inguinoscrotal descent. In the first step, testosterone (T) acts on androgen receptors (AR) in the inguinal region just outside the inguinal canal and along the mammary line. These androgen-sensitive cells of the mammary line produce a trophic factor that alters the genitofemoral nerve development to produce CGRP, which stimulates and regulates gubernacular migration
Migration of the gubernaculum through the mammary fat pad requires remodelling of the tissues. We have recently identified that there are some matrix enzymes which are present in the mammary fat pad of the male that are likely to be involved in remodelling to allow gubernacular migration [Churchill et al. 2011]. Once the gubernaculum has reached the scrotum further remodelling is required so that the gubernaculum will become attached to the inside of it. In addition, the proximal processus vaginalis normally obliterates so that the testis can reside in a separate peritoneal cavity within the scrotum.
Patho-Embryology of Abnormal Testicular Descent
Failure of the swelling reaction during the first phase of testicular descent is a relatively rare cause of cryptorchidism. As mentioned previously, only a small number of children with cryptorchidism have been identified with mutations of the gene for INSL3 [14]. In addition, the mechanical process during the first phase of descent is relatively simple, merely requiring stimulation of the gubernaculum to enlarge, thereby anchoring the testis to the future inguinal canal. Given the relative simplicity of these mechanical events, it is not surprising that abnormalities of this process are a relatively rare cause of undescended testis.
By contrast, abnormalities of the inguinoscrotal phase of testicular descent would be expected to be common, given the complexity of the migration process and the large number of regulatory factors that must be involved. The precise location of the defects which lead to undescended testis in most children remain unknown, but we would anticipate that they will reside in the complex migratory process. Further research is required in this area to identify the abnormalities. Finally, failure of remodelling of the connective tissue after migration of the gubernaculum, to allow it to become attached to the inside of the scrotum, may predispose to perinatal torsion.
Anatomy of the Testis
The inguinal canal is formed by the abdominal muscles differentiating around the gubernaculum, which is embedded in the abdominal wall at the site of the future canal [34, 35]. Initially, the inguinal canal is straight, but becomes progressively oblique as the abdominal wall enlarges. During childhood, the inguinal canal remains only 1 cm in length until about 10 years of age, when it begins to elongate further with pubertal development causing changes in the shape of the pelvis [36]. After migration of the gubernaculum is complete, the remaining mesenchyme differentiates into the fibrous layers of the spermatic cord. The processus vaginalis should have obliterated fully by 6 months of age [37], which allows the spermatic cord to elongate in proportional to growth of the boy. The failure of the processus vaginalis to obliterate completely prevents elongation of the spermatic cord, leading to an acquired undescended testis [37, 38]. Acquired cryptorchidism has remained a controversial issue [39–42] (Fig. 19.5) but there is increasing general agreement that it is common [43].
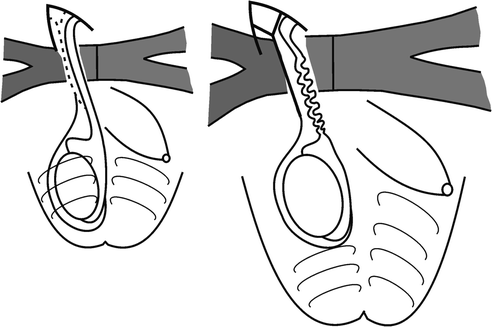

Fig. 19.5
In acquired cryptorchidism the persistence of the obliterated processus vaginalis (or an abnormal cremaster muscle in cerebral palsy) prevents normal elongation of the spermatic cord between birth and puberty. The net effect is the appearance of a descended testis gradually retracting out of the scrotum, when the testis is probably just stationary as the scrotum grows further away from the groin with age
The scrotal testis remains within its satellite peritoneal cavity, the tunica vaginalis, on a short mesentery, the mesorchium. This provides the testis with considerable mobility, but the mesentery is short enough to prevent testicular torsion. In boys where the mesentery of the testis is abnormally long there is a higher risk of torsion, which is most likely after the onset of puberty when the testis has enlarged.
When the testis fails to descend, it is important to remember that the primary abnormality is failure of gubernacular migration. This leaves the testis in the groin, but still within its tunica vaginalis, so that it is quite mobile within its peritoneal cavity. The external inguinal ring is usually quite a narrow opening after the testis has passed through it, however, when the testis is inside the inguinal canal the external ring is usually widely open. This can be determined on physical examination as a palpable triangular defect in the external oblique aponeurosis.
In most instances the cryptorchid testis is relatively normal, but becomes progressively dysplastic with the passage of time, secondary to the detrimental effects of the abnormal high temperature. In only a small percentage of cases is the testis itself abnormal, such as in children with disorders of sex development with primary testicular dysplasia.
Physiology of the Testis
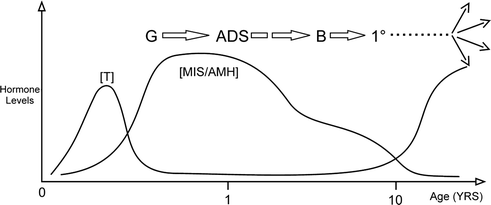
During foetal development the testis produces several hormones to control its own development. These include MIS/AMH to trigger regression of the Müllerian ducts, INSL3 to stimulate the swelling reaction of the gubernaculum, and testosterone to stimulate regression of the cranial suspensory ligament and migration of the gubernaculum (see Fig. 19.1). Initially hormone production is independent of the hypothalamic-pituitary axis, and was thought to be autonomous. However, there is now evidence that the placenta may stimulate testicular hormones between 8 and 15 weeks of development [44].
After birth the testis is initially quiescent, but after about 2 months of age it begins responding to hypothalamic and pituitary hormones [45, 46]. This leads to postnatal surges in testosterone and MIS/AMH, and possibly other hormones, which collectively control postnatal germ cell development (Fig. 19.6). At birth the germ cell in the testis is a neonatal gonocyte, which resides in the centre of the tubule. Somewhere between 3 and 6 months of age, the gonocyte transforms into a type-A spermatogonium, which resides under the Sertoli cells on the basement membrane of the testicular tubule. Over the next 3 to 4 years, the spermatogonia mature further and eventually migrate back into the centre of the tubule where they become primary spermatocytes. The primary spermatocytes thereafter remain quiescent until puberty, when they mature further into spermatids with the onset of sexual maturation.