Fig. 12.1
Incidence and number of men diagnosed with testicular cancer, 2000–2012, SEER18, by single year of age and race/ethnicity (Data from United States National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program [2])
The AYA age interval is the most dynamic of all ages in the composition of histologic types of testicular cancer and their dependence on age. In the United States, mixed germ cell tumors, rare in boys, strikingly increased with age to become the dominant type by age 15, which was replaced by middle age with seminoma as the predominant type (Fig. 12.2). Testicular choriocarcinoma was most common in AYAs and older boys (Fig. 12.2). Teratomas of the testis had a similar age distribution as choriocarcinoma except that it was far more common in male infants which may have included components of choriocarcinoma (Fig. 12.2).
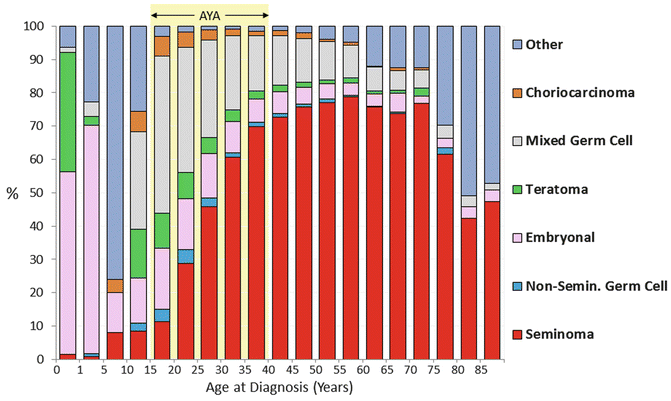

Fig. 12.2
Histology distribution of testicular cancer, 2000–2011, SEER18, by age (Data from United States National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program [2])
The majority of men present with localized or regional disease, with a notable exception in children in whom local and distant metastases are rarely detected. In the United States, the age group with the highest incidence of distant metastases at diagnosis were 10- to 20-year-olds (Fig. 12.3).
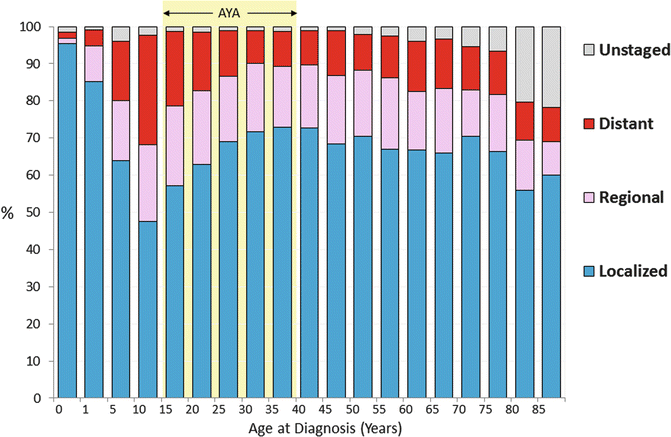

Fig. 12.3
Distribution of stage of testicular cancer, SEER 18, 2000–2011, by age (Data from United States National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program [2])
The incidence of testicular cancer has been relatively stable except for Hispanics. Since 1992 in the United States, Hispanic AYA males have had the greatest increase in incidence of testicular cancer, with no evidence for an increase in younger or older Hispanics (Fig. 12.4) [3]. Non-Hispanic whites had an increase in incidence in testicular cancer that subsided in the late 1990s.
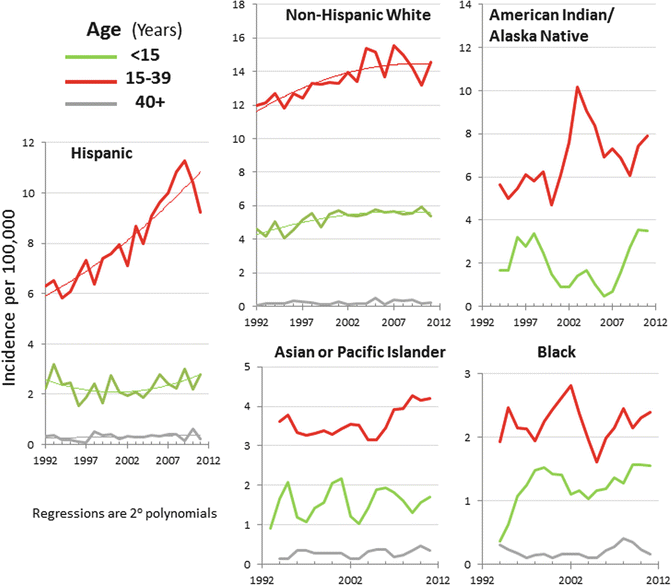

Fig. 12.4
Annual incidence of testicular cancer, 1992–2011, SEER13, by race/ethnicity, age, and year of diagnosis (Data from United States National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program [2])
12.2.1 Survival and Mortality
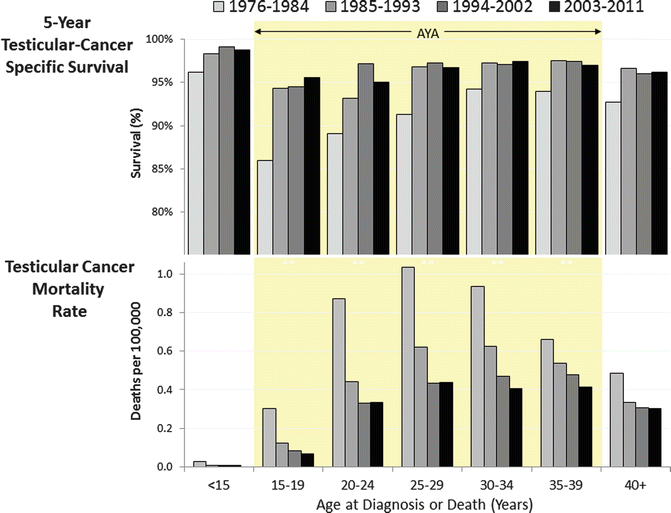
With modern therapy, testicular cancer represents a highly curable malignancy. In the 1970s and early 1980s, 15- to 19-year-olds had the worst 5-year testicular cancer-specific survival of all age groups. Since then, they have caught up, although 15- to 24-year-olds still lag behind younger and older patients (Fig. 12.5, upper panel) [4]. The latter is explained by the higher rate of metastatic disease in 15- to 19-year-olds than in all other age groups (Fig. 12.3). Deaths from testicular cancer have dramatically declined in all AYA age groups, although in 20- to 29-year-olds, the improvement ceased since the mid-1990s (Fig. 12.5, lower panel).
Among AYAs, choriocarcinoma and other non-seminomatous germ cell histologic types of testicular cancer had the worst prognosis when the distant disease was present at diagnosis, with one of each three males still dying of testicular cancer in 5 years (Fig. 12.6).
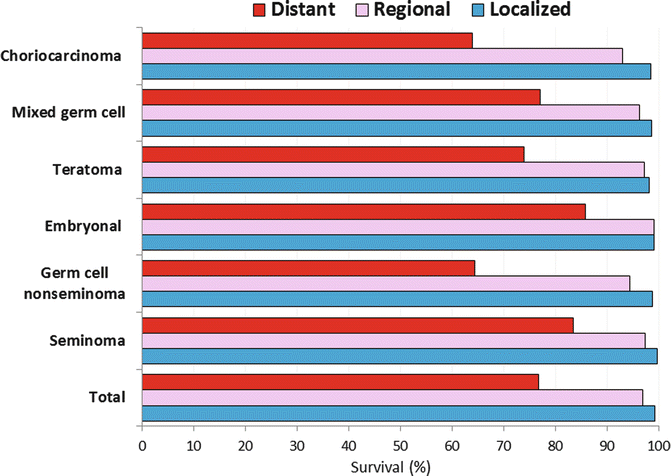

Fig. 12.6
5-year testicular cancer-specific survival, age 15–39, by histology and stage SEER 18, 2000–2010 (Data from United States National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program [4])
12.3 Biology
The vast majority of testicular tumors among AYAs are germ cell tumors, representing distinctive biology. Germ cell tumors are felt to arise from a precursor lesion of intratubular germ cell neoplasia within seminiferous tubules. Comparison of transcription factor expression with embryonic stem cells suggests the cell of origin is a pluripotent gonocyte [5]. The developmental migration of these cells along the gonadal ridge to the iliac fossa and finally to the scrotum explains the various patterns of mediastinal, retroperitoneal, and testicular presentation of germ cell tumors.
Testicular germ cell cancers are histologically and clinically divided into seminoma and non-seminoma. Seminoma is the most common histology after the age of 35. Among testicular cancer patients with a history of cryptorchidism, seminoma accounts for 60 % of tumors. Based on clinical outcome differences, only tumors with pure seminomatous features are categorized as seminoma, and tumors with mixed features (including those associated with any elevation in serum AFP) are treated as non-seminomas. A clinically distinct subtype of spermatocytic seminoma rarely metastasizes and is almost always cured with orchiectomy alone.
Non-seminoma includes all of the other histologic subtypes of germ cell tumor including yolk sac tumor, choriocarcinoma, embryonal cell carcinoma, and teratoma. While yolk sac elements are present in approximately half of non-seminomatous germ cell tumors, the pure form of yolk sac tumor is rarely seen in postpubertal males. Progression from precursor lesions to invasive germ cell tumor among pubertal and postpubertal males is often associated with acquisition of excess genetic material from the short arm of chromosome 12, including isochromosome 12p [6]. This is in contrast to prepubertal males who develop pure yolk sac tumors that are commonly diploid or tetraploid [7]. Yolk sac elements are responsible for tumor production of alpha-fetoprotein (AFP). Choriocarcinoma is a less common element of germ cell tumors and rarely seen in its pure form but is associated with an aggressive course of pulmonary and extrapulmonary metastases, including the brain. The diagnosis requires a combination of cytotrophoblasts and syncytiotrophoblasts, the latter associated with production of human chorionic gonadotropin (HCG). Embryonal cell carcinoma elements are present in up to 90 % of germ cell tumors and are associated with high-level elevations in HCG. Finally, teratoma is a tumor that contains elements of endoderm, mesoderm, and ectoderm layers. The pure form of teratoma is also rare, most often seen in children under the age of 4 years.
The development of testicular germ cell tumor is likely related to a genetic predisposition and/or environmental event in fetal development based on associations with a history of cryptorchidism (including the contralateral testis), testicular atrophy, hypogonadism, infertility, testicular dysgenesis syndromes, inguinal hernias, and first-degree relatives with testicular cancer [8]. Other evidence linking genetic events includes associations with syndromes such as the dysplastic nevus syndrome or Klinefelter’s syndrome, the latter linked to a higher incidence of germ cell tumors of mediastinal primary [9, 10].
12.4 Clinical Management
Testicular cancer should be the first consideration in evaluating a painless mass within the testis in a man, as this is the most common presentation. Prompt evaluation with ultrasound with help distinguish a testicular tumor from other abnormalities of the scrotum. Baseline serum tumor markers may assist in making a diagnosis of a testicular germ cell tumor and include AFP, HCG, and lactate dehydrogenase (LDH). Guidelines for the use of these tumor markers in adults with germ cell tumors have been published by the American Society of Clinical Oncology [11]. When a testicular mass is confirmed, the diagnostic procedure is a radical inguinal orchiectomy. Trans-scrotal biopsy should not be performed as seeding the scrotum with malignant cells may lead to a disruption in the pattern of regional lymphatic drainage or spread and compromise surveillance and surgical therapy. 25–35 % of young men with testicular cancer present with signs of metastatic disease, including abdominal or back pain, cough or hemoptysis, and headache. Fewer men are presenting with advanced metastatic disease over the past decade [12]. Due to predictable patterns of lymphatic spread, staging is completed with abdominal CT scan, chest x-ray or CT scan, and, in symptomatic patients, a brain MRI.
12.4.1 Prognostics
Significant differences in the approach to staging and prognostication exist between the pediatric and adult oncology fields, highlighting the need for increased cooperation across these subspecialties to harmonize the approach to caring for young adults with cancer. The pediatric staging system, outlined in the protocols of the Children’s Oncology Group (COG), is listed in Table 12.1. In contrast, the adult staging system, outlined by the American Joint Committee on Cancer clinical staging, is listed in Table 12.2 [13]. The AJCC clinical staging utilizes the TNM system and limits the stages of testicular cancer to I–III, whereas the pediatric system utilizes four stages.
Table 12.1
Children’s oncology group guidelines for staging of testicular germ cell tumors
Stage | Extent of disease |
|---|---|
I | Limited to the testis (testes), completely resected by high inguinal orchiectomy; no clinical, radiographic, or histologic evidence of disease beyond the testes. Patients with normal or unknown tumor markers at diagnosis must have a negative ipsilateral retroperitoneal node sampling to confirm stage I disease if radiographic studies demonstrate lymph nodes >2 cm |
II | Trans-scrotal biopsy; microscopic disease in the scrotum or high in spermatic cord (<5 cm from proximal end). Failure of tumor markers to normalize or decrease with an appropriate half-life |
III | Retroperitoneal lymph node involvement, but no visceral or extra-abdominal involvement. Lymph nodes >4 cm by CT or >2 cm and <4 cm with biopsy proof |
IV | Distant metastases, including the liver |
Table 12.2
The American Joint Committee on Cancer clinical staging for testicular germ cell tumors
Tumor (pathologic) | T1: Limited to the testis/epididymis without lymphovascular invasion. May invade tunica albuginea but not tunica vaginalis |
T2: Limited to the testis/epididymis with lymphovascular invasion or extending to involve tunica vaginalis | |
T3: Invades the spermatic cord with or without vascular/lymphatic invasion | |
T4: Invades the scrotum with or without vascular/lymphatic invasion | |
Node | N1: Lymph node mass ≤2 cm in greatest dimension or multiple lymph nodes, not >2 cm in greatest dimension |
N2: Lymph node mass >2 cm but not >5 cm in greatest dimension, or multiple lymph nodes, any mass >2 cm but not >5 cm in greatest dimension | |
N3: Lymph node mass >5 cm in greatest dimension | |
Metastasis | M1a: Non-regional lymph node or pulmonary metastasis |
M1b: Distant metastasis other than to non-regional lymph node and lungs | |
Stage | Extent of disease |
I
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|