Target
Agent(s)
Phase
Summary
Identifier
mTOR
Everolimus
(Docetaxel)
(Bevacizumab)
Ib/II
Dose-finding/efficacy study;
Docetaxel + everolimus + bevacizumab in metastatic CRPC
NCT00574769
mTOR
Temsirolimus (Bevacizumab)
I/II
Dose-finding/efficacy study;
Temsirolimus + bevacizumab in metastatic CRPC
NCT01083368
mTOR
Everolimus
(Docetaxel)
I/II
Dose-finding/efficacy study;
Docetaxel + everolimus in metastatic CRPC
NCT00459186
mTOR
Temsirolimus
(Cixutumumab)
I/II
Dose-finding/efficacy study;
Temsirolimus + cixutumumab (IGF-1R antibody) in metastatic CRPC
NCT01026623
mTOR
AKT
Ridaforolimus MK2206
(MK0752)
I
Dose-finding study;
Ridaforolimus + MK2206 or Ridaforolimus + MK0752 (Notch inhibitor) in metastatic CRPC
NCT01295632
PI3K +
mTOR
BEZ235
(Abiraterone)
I/II
Dose-finding/efficacy study;
BEZ235 + abiraterone in metastatic CRPC
NCT01717898
PI3K +
mTOR
BEZ235
BKM120
(Abiraterone)
Ib
Dose-finding study;
Abiraterone + BEZ235 or Abiraterone + BKM120 in CRPC
NCT01634061
AKT
MK2206
(Bicalutamide)
II
Randomized efficacy study;
Bicalutamide +/− MK2206 in PSA-recurrent (non-metastatic) prostate cancer
NCT01251861
PI3K
BKM120
II
Single-arm efficacy study;
BKM120 in metastatic CRPC
NCT01385293
PI3K
BKM120
(Abiraterone)
Ib
Single-arm efficacy study;
Abiraterone + BKM120 in metastatic CRPC
NCT01741753
PI3K
PX-866
II
Single-arm efficacy study;
PX-866 in metastatic CRPC
NCT01331083
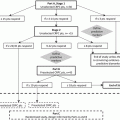
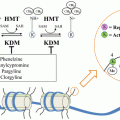
One possible explanation for the inability of single-agent mTOR inhibitors to show efficacy in prostate cancer is the hypothesis that mTOR blockade leads to feedback-driven upregulation of signaling molecules upstream in the PI3K pathway. For example, rapamycin and rapalogs are primarily inhibitors of mTORC1 (mTOR in complex with raptor) but not mTORC2 (mTOR in complex with raptor). This might lead to compensatory phosphorylation of S473, one of the activation sites of AKT. Thus, there is a theoretical advantage of utilizing active site inhibitors of mTOR [11]. Seminal research by Carver et al. [12] has demonstrated the existence of bidirectional cross-talk between the PI3K pathway and androgen receptor (AR) signaling. For example, in a preclinical model, inhibition of the PI3K pathway resulted in activation of AR signaling in PTEN-deficient prostate cancer cells. Conversely, the AR antagonist enzalutamide appeared to upregulate AKT signaling by reducing levels of the regulatory phosphatase PHLPP. Moreover, combined blockade with the dual PI3K/mTOR inhibitor, BEZ235, administered together with enzalutamide led to reductions in tumor size in xenograft models of human prostate cancer that exceeded the effects seen with either agent used alone [12]. This work provides a sound rationale for simultaneous targeting of the androgen/AR pathway and the PI3K/mTOR pathway, a discovery that is beginning to be translated into the clinic (Table 17.1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree