The Hedgehog (Hh) pathway is a signaling cascade that is evolutionally highly conserved and plays an important role in embryonic pattern formation and stem cell response to tissue damage. Given the pivotal role the Hh pathway plays in embryonic development in terms of proliferation and differentiation, it is not surprising that it has also been implicated in tumorigenesis and tumor growth acceleration in a vast variety of malignancies. This article summarizes the mechanism of Hh pathway signal transduction, discusses the models of pathway activation, reviews the clinical data using Hh inhibitors, and discusses challenges to the development of pathway inhibitors.
The Hedgehog (Hh) pathway is a signaling cascade that is evolutionally highly conserved and plays an important role in embryonic pattern formation and stem cell response to tissue damage. The Hh gene was originally identified in 1980 by Christiane Nusslein-Volhard and Eric F. Wieschaus, developmental biologists studying the segmentation pattern of Drosophila melanogaster (fruit fly) embryos. Their genetic analysis found that loss of the gene encoding the secreted protein that is a key regulator of the pathway in fruit flies gives rise to a mutant embryonic phenotype with spiky projections similar to the appearance of a hedgehog. Three Hh homolog genes have since been identified in vertebrates and are termed Sonic hedgehog (Shh) after the popular video game character, and Indian hedgehog (Ihh) and Desert hedgehog (Dhh), both after existing species of hedgehogs. The Hh family of proteins serve a variety of functions in vertebrate embryogenesis, including body patterning, cell migration, growth, differentiation, and survival. This signaling is context dependent, varying based on the receiving cell type, can be short range and long range, and is concentration dependent, typical of a morphogen. The fundamental regulatory role Hh plays in development is underscored by the consequences of pathway misregulation or inhibition, which have been shown to cause severe birth defects, including holoprosencephaly, cyclopia, and microcephaly. In adults, the pathway is much less active and widespread. It is mainly involved in tissue maintenance and repair, including the skin, bone, and intestine.
- •
The Hh pathway is a vital signaling pathway in embryogenesis and more recently has been implicated in the pathogenesis of a vast variety of malignancies.
- •
The key components of the pathway include the Hh ligands Sonic, Indian, and Desert, which bind the receptor PTCH, thereby relieving its inhibition of the receptor SMO, leading to downstream signaling via the GLI transcription factors.
- •
There are multiple models for aberrant Hh pathway activation, including both ligand dependent and independent, as well as a role in tumor stem cell maintenance.
- •
Hh pathway inhibition is an active area of research, with strong preclinical rationale and proof of concept in malignancies, such as basal cell carcinoma, which have constitutive activation of the Hh pathway.
- •
Novel pathway inhibitors have been or are currently being developed to antagonize the Hh pathway and provide a molecularly targeted approach to cancer treatment.
Given the pivotal role the Hh pathway plays in embryonic development in terms of proliferation and differentiation, it is not surprising that it has also been implicated in tumorigenesis and tumor growth acceleration in a vast variety of malignancies. The initial connection between aberrant Hh pathway signaling and cancer was made in a rare condition called Gorlin syndrome, in which patients develop numerous basal cell carcinomas (BCCs) during their lifetimes and are predisposed to other types of malignancies, including medulloblastoma and rhabdomyosarcoma. These patients were noted to have mutations in components of the Hh pathway, leading to its constitutive activation. Other types of cancers, including for example breast, prostate, pancreas, colon, lung, and chronic myelogenous leukemia (CML), have subsequently been shown to have inappropriate Hh pathway activation. Given that misregulation of the Hh pathway has been implicated in such a wide array of disparate malignancies, targeting its signaling has been an active area of research over the past 2 decades. In this article, I summarize the mechanism of Hh pathway signal transduction, discuss the models of pathway activation, review the clinical data using Hh inhibitors, and discuss challenges to the development of pathway inhibitors.
Pathway signal transduction
Signaling within the Hh pathway begins with the secretion of 1 of the 3 Hh proteins: Sonic, Indian, or Desert. The Hh protein undergoes extensive processing, with many modifications and is an unusual secreted protein in that it harbors 2 covalently linked lipid adducts. The precursor protein undergoes initial autoprocessing to release its N-terminal fragment. This peptide is subsequently modified by covalent binding of a cholesterol molecule on its C terminus, conferring a high affinity for the plasma membrane and precluding its further release and spread. The N-terminus then undergoes palmitoylation mediated by an acyl transferase encoded by HHAT. Thus, the ultimate secreted morphogen is termed Hh-Np (“p” standing for processed). The subsequent secretion of the processed protein involves several molecules for its movement, extracellular transport, and release—the best characterized being the large multipass transmembrane protein Dispatched (DISP). It appears that DISP is essential for Hh signaling, as animals with mutations in DISP have accumulation of Hh in producing cells and therefore downstream signaling of the pathway is lost.
Once secreted from the producing cell, Hh binds on the receiving cell to the Patched protein (PTCH), a 12-transmembrane protein, and initiates the signaling cascade. The simplest model is that Hh binds PTCH, alleviating PTCH inhibition of the 7-transmembrane G-protein-coupled receptor–like protein Smoothened (SMO), thereby enabling signal transduction to the nucleus ( Fig. 1 ). Conversely, in the absence of ligand binding, PTCH catalytically inhibits the activity of SMO; however, the exact mechanism of the interaction is unclear. It is known from tissue-cultured cells that PTCH inhibits SMO at substoichiometric concentrations. One proposed mechanism for PTCH inhibition of SMO may involve the transport by PTCH of a small molecule regulator of SMO movement or activity. Candidates for this small molecule include PI4P, lipoproteins, and provitamin D3. Another possible mechanism is that PTCH represses SMO by affecting its localization to the cell surface. Specifically, it is thought that PTCH prevents SMO translocation into the primary cilium, a microtubule-based antennalike structure that emanates from the surface of virtually all cells in the mammalian body. The primary cilium is a sensory organelle that receives both mechanical and chemical signals from other cells and the environment, and transmits these signals to the nucleus to elicit a cellular response. It has been shown to play a pivotal role in the Hh transduction pathway. For example, mutations in intraflagellar transport, which normally functions in retrograde and anterograde movement of molecules within the primary cilium, results in mice with Hh loss-of-function phenotypes. When exposed to Hh ligand, PTCH, which is normally present in the cilia, becomes internalized, thereby allowing SMO to move from internal vesicles all the way up along the shaft of the cilium.
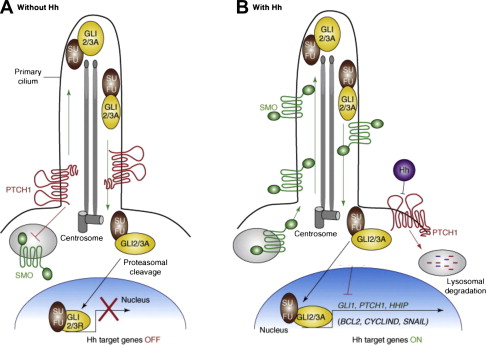
Once Hh binds to PTCH and thereby alleviates repression of SMO, a signaling cascade is initiated through activation of the GLI family of zinc transcription factors, thereby translating the extracellular stimulus into defined transcriptional programs in a context-dependent and cell-type specific manner. Unlike Drosophila , which have a single GLI protein that is encoded by the cubitus interruptus gene, vertebrates have 3 GLI transcriptions factors (GLI 1–3). GLI2 and 3 are bifunctional transcription factors, functioning as either activators or repressors depending on their modification, processing, and nuclear trafficking. Because GLI1 lacks the N-terminal repressor domain, it functions exclusively as an activator. It is also most highly dependent on active Hh signaling and therefore is the most often used surrogate for pathway activation. GLI family members are phosphorylated by protein kinase A (PKA) and glycogen synthase 3 (GSK3) in the absence of Hh signaling, but are relieved from PKA/GSK3-mediated phosphorylation in the presence of Hh signaling. In the absence of Hh activation, GLI2 is degraded, owing to phosphorylation-mediated ubiquitylation, and GLI3 is processed into a repressor. Once the Hh pathway is activated, GLI family members are stabilized, translocated into the nucleus, and bind the consensus binding site (5′-TGGGTGGTC-3′) in the target gene promoter. Hh signaling seems to be dependent on the relative balance of GLI activator and repressor forms. The activity of GLI transcription factors is regulated at several different levels, including nuclear-cytoplasmic shuttling, ubiquitination, protein degradation, and the transcriptional activity of GLI molecules. One well-characterized negative regulator of Hh signaling is the Suppressor of Fused (SUFU) protein, which acts as a tumor suppressor gene. It associates with and inhibits GLI molecule function and is required for GLI3 processing. The Hh target genes are numerous and involved in a wide variety of cellular functions, including control of cell proliferation, survival, epithelial-to-mesenchymal transition (EMT), maintenance of stemness, and cell fate determination. In addition, GLI, PTCH, and Hh-interacting protein are not only components of the pathway, but are also target genes themselves and involved in several regulatory feedback loops.
Linking the H h pathway and human cancer
Similar to its role in Drosophila , the Hh pathway in humans was initially implicated in embryogenesis and development as an essential signaling pathway. Unlike in the embryo or neonate, Hh activation was found to be much more limited in the adult and restricted to areas of tissue damage and repair. More recently, however, aberrant Hh pathway signaling has been implicated in cancer formation and growth. The link was first made in 1996 when mutations in PTCH were discovered to be associated with a rare hereditary form of BCC, called basal cell nevus syndrome or Gorlin syndrome. Gorlin syndrome is an autosomal dominant genetic disease in which patients develop numerous BCCs during their lifetime and are at an increased risk of other tumors, including medulloblastoma, a tumor of the cerebellar progenitor cells, and rhabdomyosarcoma, a muscle tumor. These patients have a germline mutation in 1 allele of the PTCH gene, and basal-cell tumors from these patients lack the remaining normal PTCH gene. Without functional PTCH, SMO is freed from repression and the Hh pathway becomes constitutively active. The association between the Hh pathway and tumorigenesis was further supported by the identification of aberrant activation in sporadic BCCs. Most BCC tumors have either inactivating mutations in PTCH1 (>85%) or, less commonly, activating mutations in SMO. Since the initial connection between the Hh pathway and carcinogenesis in BCCs, aberrant signaling and/or reactivation of the cascade have been noted in a wide and disparate number of cancers, including both solid tumor and hematologic malignancies.
Linking the H h pathway and human cancer
Similar to its role in Drosophila , the Hh pathway in humans was initially implicated in embryogenesis and development as an essential signaling pathway. Unlike in the embryo or neonate, Hh activation was found to be much more limited in the adult and restricted to areas of tissue damage and repair. More recently, however, aberrant Hh pathway signaling has been implicated in cancer formation and growth. The link was first made in 1996 when mutations in PTCH were discovered to be associated with a rare hereditary form of BCC, called basal cell nevus syndrome or Gorlin syndrome. Gorlin syndrome is an autosomal dominant genetic disease in which patients develop numerous BCCs during their lifetime and are at an increased risk of other tumors, including medulloblastoma, a tumor of the cerebellar progenitor cells, and rhabdomyosarcoma, a muscle tumor. These patients have a germline mutation in 1 allele of the PTCH gene, and basal-cell tumors from these patients lack the remaining normal PTCH gene. Without functional PTCH, SMO is freed from repression and the Hh pathway becomes constitutively active. The association between the Hh pathway and tumorigenesis was further supported by the identification of aberrant activation in sporadic BCCs. Most BCC tumors have either inactivating mutations in PTCH1 (>85%) or, less commonly, activating mutations in SMO. Since the initial connection between the Hh pathway and carcinogenesis in BCCs, aberrant signaling and/or reactivation of the cascade have been noted in a wide and disparate number of cancers, including both solid tumor and hematologic malignancies.
Models of pathway activation in cancer
There are 3 basic models that have been proposed for Hh pathway activity in cancer ( Fig. 2 ). The first elucidated was the Hh ligand-independent cancers, such as BCCs, and were termed type I cancers. These tumors have pathway-activating mutations that lead to constitutive activation of the Hh pathway. Type II cancers are ligand-dependent and autocrine (or juxtacrine), so that Hh is both produced and responded to by the same (or neighboring) tumor cells, whereas Type III cancers are also ligand dependent, but use paracrine signaling. In this model, Hh is produced by the tumor epithelium and received by the surrounding stroma (analogous to epithelial-to-mesenchymal transition in early development), which then feeds back growth and/or survival signals to the tumor. A variation to this model has recently been described, termed “reverse paracrine” signaling or Type IIIb, where Hh is secreted from stromal cells to the receiving cells in a tumor.
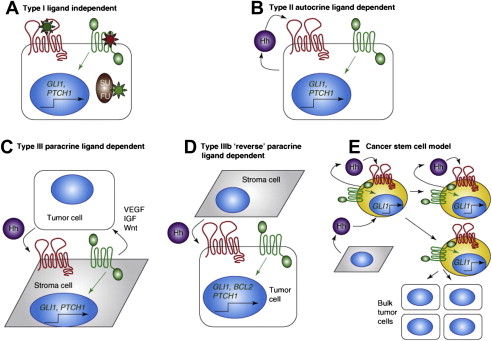
In addition to the aforementioned 3 models, there is an alternative model that implicates aberrant Hh pathway activation in cancer stem cells (CSCs). CSCs comprise a small subset of cells in a tumor that are characterized by being able to self-renew and initiate tumor spread. They are typically resistant to chemotherapy, possibly contributing to relapse. Similar to its role in normal stem cell activity, the Hh pathway has been shown to be active in several types of CSCs, including breast, glioma, pancreatic, multiple myeloma, and CML. The exact mechanism of Hh deregulation within CSCs is still unclear and may rely on autocrine, paracrine, and/or ligand-independent signaling. What is clear, however, is that Hh inhibition appears to deplete this tumorigenic population in animal models and therefore holds great potential promise in the clinic.
Discovery of the first H h inhibitor: cyclopamine
The discovery of the first Hh pathway inhibitor is an interesting story and dates back to the 1950s when an increased incidence of congenital malformations, including cyclopia, was noted in newborn lambs. These teratogenic effects were linked to pregnant ewes consuming a corn lily plant ( Veratrum californicum ). Analysis of the plant led to isolation and identification of the principal teratogen, termed cyclopamine, a steroidal alkaloid. Following the discovery of the Hh pathway first in Drosophila and later in humans and the understanding that mutations within this signaling cascade can lead to deformities, such as holoprosencephaly, which can include cyclopic features, Cooper and colleagues demonstrated that cyclopamine acts through inhibition of the Hh pathway. The elucidation of cyclopamine as an Hh pathway inhibitor took decades and has since served as a powerful tool to help understand the role the Hh pathway plays in diseases, including cancer.
Cyclopamine inhibits the Hh pathway by binding to, and inactivating SMO. It has been used extensively in preclinical studies; however, because of its low affinity, poor oral bioavailability, and suboptimal pharmacokinetics, it has not moved forward in the clinic. Furthermore, in addition to inactivating SMO, cyclopamine may also have “off-target” effects, as cells without the SMO receptor have been shown to undergo apoptosis and often very high concentrations of cyclopamine are required to inhibit cell proliferation. Given the multiple impediments to the clinical use of cyclopamine, as well as the potential importance of the Hh pathway in a vast array of pathologies, additional inhibitors with improved pharmacologic properties have since been identified via high-throughput screening of small-molecule libraries. Although the screens were designed to probe the entire Hh signaling cascade, most of the identified inhibitors have been reported to target SMO itself. SMO seems to be the most druggable component of the pathway, whereas other components are more structurally complex and inaccessible.
H h pathway inhibitors
Among the various Hh pathway inhibitors (HPIs), vismodegib (GDC-0449; Curis/Genentech) is the farthest along in development. Vismodegib is a synthetic small-molecule inhibitor of the Hh pathway that binds to and inhibits SMO. It was designed to be more potent and have more favorable pharmaceutical properties than the steroidal alkaloid cyclopamine. Phase I clinical testing is completed and vismodegib is now being evaluated in multiple phase II studies, some of which have recently been reported, including colon, ovarian, and BCCs (see later in this article). Other synthetic SMO inhibitors include BMS-833923 (XL139; Exelexis/Bristol-Myers Squibb), LDE225 and LEQ506 (Novartis), PF-04449913 (Pfizer), and LY2940680 (Eli Lily), which have either completed or are nearing completion of phase I testing. IPI-926 (Infinity) is the only semisynthetic SMO inhibitor currently in clinical testing. It was derived from cyclopamine and demonstrates improved potency and physiochemical properties, including acid stability and aqueous solubility. Phase I single-agent findings have been reported and currently combination phase I and phase II testing is ongoing for chondrosarcoma, and pancreatic and head and neck cancers ( Table 1 ).
| Drug | Tumor Target | Other Agent(s) | Phase | Design | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| GDC-0449 | Pancreas | — | II (single arm) | Preoperative GDC-0449 | NCT01096732 |
| Pancreas | Gemcitabine, nab-paclitaxel | II (single arm) | Metastatic disease treated with GDC-0449 + chemotherapy | NCT01088815 | |
| Pancreas | Gemcitabine | Unspecified | Pilot study to investigate pancreatic stem cells before & after treatment with GDC-0449 + gemcitabine | NCT01195415 | |
| Pancreas | Erlotinib, gemcitabine | I | Metastatic disease treated with GDC-0449 + erlotinib ± gemcitabine | NCT00878163 | |
| Pancreas | Gemcitabine | II (randomized, placebo controlled) | Metastatic disease treated with gemcitabine ± GDC-0449 | NCT01064622 | |
| Small cell lung cancer | Cisplatin (cis), etoposide (E) | II (randomized) | Three arms: cis+E, cis+E+ GDC-0449, cis+E + cixutumumab | NCT00887159 | |
| Gastroesophageal | 5-Fluoruracil, oxaliplatin | II (randomized, placebo controlled) | Advanced gastroesophageal cancer treated with FOLFOX ± GDC-0449 | NCT00982592 | |
| Prostate | Leuprolide acetate or goserelin | Ib/II (randomized) | Preoperative GDC-0449 + androgen ablation compared with androgen ablation alone followed by radical prostatectomy for locally advanced prostate adenocarcinoma | NCT01163084 | |
| Medulloblastoma | — | I | Recurrent or refractory medulloblastoma in pediatric patients | NCT00822458 | |
| Medulloblastoma | — | II (single arm) | Recurrent or refractory medulloblastoma in pediatric patients, stratified by IHC for Hh pathway | NCT01239316 | |
| Medulloblastoma | — | II (single arm) | Recurrent or refractory disease treated with GDC-0449 | NCT00939484 | |
| Sarcoma | RO4929097 | Ib/II | Advanced or metastatic sarcomas treated with combination GDC-0449 + gamma secretase inhibitor | NCT01154452 | |
| Chondrosarcoma | — | II | Metastatic or unresectable chondrosarcoma treated with GDC-0449 | NCT01267955 | |
| Multiple myeloma | — | Ib | High-risk myeloma in first remission following autologous stem cell transplantation | NCT01330173 | |
| Basal Cell Carcinoma (BCC) | — | II | 2-cohort trial evaluating the efficacy & safety of GDC-0449 in operable BCC | NCT01201915 | |
| BCC | — | II (single arm) | Locally advanced or metastatic BCC | NCT01367665 | |
| IPI-926 | Pancreas | Gemcitabine | Ib/II | Metastatic disease patients randomized in phase II to gemcitabine ± IPI-926 | NCT01130142 |
| Pancreas | 5-Fluorouracil, irinotecan, oxaliplatin (FOLFIRINOX) | I | Advanced disease treated with FOLFIRINOX + IPI-926 | NCT01383538 | |
| Head and neck | Cetuximab | I | Advanced disease treated with combined cetuximab + IPI-926 | NCT01255800 | |
| Chondrosarcoma | — | II (randomized, placebo controlled) | Unresectable disease treated with IPI-926 or placebo | NCT01310816 | |
| Myelofibrosis | — | II | Single-arm study with primary end point of response rate | NCT01371617 | |
| LDE225 | BCC | — | II | Randomized double-blind study of efficacy and safety of 2 dose levels of LDE225 in unresectable disease | NCT01327053 |
| BCC | — | II (randomized, placebo controlled) | Adult patients with nevoid BCC syndrome | NCT01350115 | |
| Pancreas | Gemcitabine | I/II | Neoadjuvant combination therapy in borderline resectable pancreatic cancer | NCT01431794 | |
| BMS-833923 | CML | Dasatinib | I | Dose-finding study in CML patients with resistance or suboptimal response to a prior TKI | NCT01218477 |
| Gastroesophageal | Cisplatin, capecitabine | Ib | 1st line treatment of advanced disease | NCT00909402 | |
| Small cell lung cancer (SCLC) | Carboplatin, etoposide | Ib | Extensive stage SCLC treated with BMS-833923 + carboplatin + etoposide followed by maintenance BMS-833923 | NCT00927875 | |
| PF-04449913 | CML/hematologic malignancies | Dasatinib or bosutinib | I | PF- 04449913 in select hematologic malignancies or in combination with dasatinib or bosutinib in CML | NCT00953758 |
| Solid tumors | — | I | Advanced solid tumors treated with single agent | NCT01286467 | |
| LY2940680 | Solid tumors | — | I | Advanced solid tumors treated with single agent | NCT01226485 |
| Itraconazole | BCC | — | Pilot | To determine if 3 weeks of oral or topical itraconazole reduces BCC biomarkers | NCT01108094 |
| SCLC | Pemetrexed | Randomized phase II | Previously treated SCLC treated with pemetrexed ± itraconazole | NCT00769600 |
Although there are definite differences among the various SMO inhibitors in terms of structure, they otherwise have similar profiles. They are all oral selective small molecule inhibitors with high potency (half maximal effective concentration <20 nM), long half-lives (24–168 hours), and a very manageable side-effect profile. The most common toxicities include fatigue, dysgeusia, myalgias, alopecia, and nausea, which may be a class effect. They also have all shown to have exposure-dependent target inhibition as measured by GLI expression.
Apart from SMO inhibitors, there are other Hh pathway components that are being targeted for inhibition. One of the more challenging targets has been the Hh pathway ligand Shh, an extracellular protein that is upstream of SMO and undergoes extensive lipid modification, making it a challenge to access. The macrocylic small molecule robotnikinin is the first reported inhibitor of Shh. It was discovered through a small-molecule microarray-based screen of a bacterially expressed biologically active Shh N-terminal fragment (ShhN) and then subsequently optimized for better binding and dissociation time. It binds to ShhN at concentrations between 1.56 and 25.00 μM with a Kd value of 3.1 μM. It was shown to block transcription of Hh pathway targets in primary keratinocytes treated with Shh compared with that seen in mock-treated controls. It did not prevent transcription triggered by small-molecule agonists of SMO, suggesting that the compound acts earlier in the pathway than SMO. Although still in preclinical development, robotnikinin and other small-molecule inhibitors of Shh may ultimately play an important role in the treatment of ligand-dependent tumors. There are multiple other inhibitors that act downstream of SMO on various different facets of the Hh pathway. Inhibition of GLI-mediated transcription is an attractive target, as it is theoretically efficacious irrespective of the mode of Hh pathway activation. Two low-molecular-weight compounds, GANT58 and GANT61, have been identified, which act at the nucleus to block GLI function. Although they are from different chemical classes, they both are potent and selective inhibitors of GLI with a half maximal inhibitory concentration of ∼5 μM. Furthermore, they both block cell growth in an in vivo xenograft prostate cancer model. More recently, 4 additional Hh pathway inhibitors, which act downstream of SMO, have been characterized. Termed HPI-1 through HPI-4, each has a unique mechanism of action, involved in GLI processing, GLI activation, and primary cilia formation, suggesting that multiple steps in GLI regulation are pharmacologically targetable.
Additionally, 2 well-known and widely used agents approved by the Food and Drug Administration (FDA) for separate indications have been shown to inhibit the Hh pathway. Both itraconazole, a commonly used antifungal, and arsenic trioxide, an important treatment for acute promyelocytic leukemia, have demonstrated preclinical evidence for Hh pathway inhibition. Arsenic trioxide’s mechanism of action is thought to be through inhibition of GLI transcriptional effectors, blocking Hh-induced ciliary accumulation of GLI2 and thereby reducing steady-state levels, whereas itraconazole has been shown to act at the level of SMO by a mechanism that is distinct from that of cyclopamine and other known SMO antagonists, and prevents the ciliary accumulation of SMO normally caused by Hh stimulation. There is currently a clinical trial under way with itraconazole in patients with BCCs, building on this new understanding of an established drug (see Table 1 ).
Ligand-independent tumors and H h inhibition
Basal Cell Carcinoma
Since the initial recognition in 1996 of the link between Gorlin syndrome and aberrant activation of the Hh pathway, much has been learned about Hh mutation–driven tumors and their treatment. Following the identification of germline-inactivating mutations in the PTCH gene in patients with Gorlin syndrome, sporadic BCCs were subsequently also found to have mutations in the Hh pathway. Further research has established that upregulation of Hh signaling is the pivotal abnormality in all BCCs. Specifically, nearly 90% have identifiable mutations in at least one allele of PTCH (loss of function mutation) and an additional 10% have activating mutations in SMO (gain-of-function mutation), with rare mutations also found in SUFU. These mutations lead to constitutive activation of the Hh pathway with increased cell proliferation and tumor formation. Various preclinical models have confirmed the role of aberrant Hh pathway activation in BCCs. For example, overexpression of SHH, GLI, or SMO in the skin of otherwise normal mice produces lesions that are BCC-like in histologic appearance and phenotypic markers. Furthermore, mice that are heterozygous for PTCH are more susceptible to UV-induced BCC. These same models have also helped to confirm the efficacy of HPIs in blocking tumor cell growth. For example, SMO antagonists have been shown to suppress proliferation and induce apoptosis of basaloid nests in BCC model systems, while having no effect on normal skin cells. Given the strong preclinical evidence demonstrating the Hh pathway as the key oncogenic pathway driving the formation and maintenance of BCCs, attempts at pathway inhibition downstream of the causative mutation were soon made in the clinic.
One of the first tested HPIs in the clinic for the treatment of BCCs was a topical SMO antagonist called CUR61414. Based on promising results with use of the topical compound in mice, a phase I randomized, double-blind placebo-controlled study was undertaken in superficial or nodular BCCs from 2005 to 2006. The primary end point was the safety and tolerability of a multidose regimen of CUR61414 at various concentrations applied topically for up to 28 days. A secondary objective included clinical activity as measured by the percentage of tumors with complete clearance. A total of 42 patients were enrolled and there were no serious adverse events (SAEs) reported. Most adverse events (AEs) were skin-related and of mild severity. In terms of response, none of the patients had a complete tumor clearance, defined as both clinical and histopathologic. Unlike in the mouse, the treatment failed to reduce GLI or PTCH expression in 8 patients tested, suggesting a differential potency in human versus mouse SMO and/or inadequate drug concentrations in BCCs owing to low penetration or rapid clearance. Although this initial study was not successful in validating Hh inhibition as a useful method for treating BCC, subsequent studies have been more promising.
The first support of proof of concept was reported by Von Hoff and colleagues in a phase I trial investigating the safety and tolerability of vismodegib in patients with a variety of solid tumors that were refractory to standard therapy. The study had an open-label, multicenter, 2-stage design in which vismodegib was dose escalated until dose-limiting toxicity, disease progression, or lack of benefit, as determined by the investigator. In the initial stage, vismodegib was escalated from 150 mg daily to 270 mg daily and then to 540 mg daily. There were no dose-limiting side effects observed, and the dosage of 150 mg daily was chosen as the recommended phase 2 dosage because higher dosages did not result in an increased plasma concentration of the drug. There were 3 patients with BCC enrolled in the first stage, 2 of whom were noted to have a clinical benefit. As a result, the second stage included a cohort of patients with advanced BCC in addition to a cohort of patients with solid tumors, a group that was enriched with patients with BCC. In total, there were 33 patients with BCC enrolled in the study. Of the 18 patients with metastatic BCC tumors, the overall response rate (RR) was 50% (95% confidence interval [CI]: 29–71), and of the 15 patients with locally advanced BCC, the RR was 60% (95% CI: 33–83). The treatment was very well tolerated, without any dose-limiting or grade 5 AEs. A single grade 4 AE (asymptomatic hyponatremia) occurred. The most common grade 2 and 3 AEs included fatigue, myalgias, weight loss, dysgeusia, and hyponatremia. Pharmacodynamic studies demonstrated down modulation of the Hh pathway as measured by a decrease in GLI1 expression in nonaffected skin biopsies taken pretreatment and on treatment. Furthermore, GLI1 mRNA was overexpressed in BCC tumors when compared with control normal skin or lung tumor ( P <.001 for all comparisons). When tumor tissue was analyzed for mutations in the PTCH1 gene, 9 of 10 specimens were found to harbor the mutation, further supporting PTCH’s role as a tumor suppressor, with its loss of activity driving oncogenesis.
This study was the first to confirm the participation of the Hh pathway in BCC and suggests that its inhibition would be a valuable treatment for inoperable BCC. As a result, a phase II, international, single-arm, multicenter, 2-cohort, open-label study was conducted in 104 patients with advanced BCC, including 71 with locally advanced (laBCC) disease and 33 with metastatic disease (mBCC). Patients received 150 mg vismodegib daily until disease progression or intolerable toxicity. The primary end point was overall response rate, as assessed by an independent review facility, with secondary end points including investigator-assessed overall response rate (ORR), progression-free survival (PFS), overall survival (OS), and duration of response in all evaluable patients. The ORR, as assessed by an independent review facility, showed vismodegib substantially shrank tumors or healed visible lesions, with an observed RR of 43% in the laBCC cohort and 30% in the mBCC cohort. Study investigators assessed the ORR to be 55%, with 60% in the laBCC cohort, and 46% in mBCC cohort. The clinical benefit rate (defined as patients who experienced response, as well as those who experienced prolonged stable disease for more than 24 weeks) showed vismodegib shrank tumors or healed visible lesions, or prevented them from growing any further in 75% of patients with laBCC and 76% of patients with mBCC, as assessed by independent review. The median duration of PFS by independent review for both patients with mBCC and patients with laBCC was 9.5 months. Treatment was generally well tolerated and the most common AEs included muscle spasms, hair loss, altered taste sensation, weight loss, fatigue, nausea, decreased appetite, and diarrhea; however, there were 4 SAEs thought to be related to treatment, including cholestasis, dehydration with syncope, pneumonia with heart failure, and pulmonary embolism. Based on these data, vismodegib has recently been FDA approved for the treatment of adults with metastatic basal cell carcinoma or with locally advanced basal cell carcinoma that has recurred following surgery or who are not candidates for surgery, and who are not candidates for radiation.
In addition to vismodegib, several other SMO antagonists have also demonstrated antitumor activity in BCC. For example, in a phase I study of LDE225 in advanced solid tumors, 1 patient with BCC experienced a complete response (CR) with histologic clearance, 4 patients with BCC had a partial response (PR), and 2 patients with BCC had disease stabilization. IPI-926 also is active in BCC, showing 6 (27%) clinical or radiologic PRs in the 22 patients with Hh-inhibitor naive BCC. In addition to clinical activity, these HPIs have demonstrated convincing pharmacodynamic data, with an exposure-dependent reduction of GLI1 mRNA, verifying that they are hitting their target within the pathway.
Given the mild, but certainly bothersome, side effects that have been reported with the various oral SMO antagonists in the treatment of BCC, topical routes continue to be investigated as a possibly less toxic and equally efficacious modality. Although the initial study of topical CUR61414 did not demonstrate any efficacy in humans, a subsequent study with a different HPI merits promise. Topical LDE225 was developed as a distinct and selective antagonist of SMO with single-digit nanomolar potency. In an exploratory study, 8 patients with nevoid basal cell carcinoma syndrome were enrolled in a double-blind, randomized, vehicle-controlled intraindividual study. Among the 8 patients, there were a total of 27 BCC tumors treated twice daily with 0.75% LDE225 cream or vehicle for 4 weeks. No skin irritation was noted or any clinically significant abnormalities. Of 13 BCCs treated with LDE225, 12 showed a clinical response (3 CR, 9 PR), whereas only 1 of the 14 lesions treated with vehicle demonstrated a clinical response. In addition, a greater than twofold downregulation of Hh target genes was demonstrated in 6 of 8 patients, confirming that the topical SMO inhibitor hits its target.
Medulloblastoma
Medulloblastoma, a rare and aggressive childhood tumor of cerebellar origin, is another malignancy that has demonstrated dependence on the Hh pathway for its pathogenesis. As with BCC, the first indication that the Hh pathway was involved in medulloblastoma came from the fact that patients with Gorlin syndrome have an increased risk of developing this unusual brain tumor, occurring in approximately 5% of these patients. The Hh pathway’s role in medulloblastoma is not surprising, given the importance of its signaling in the normal cerebellar maturation process. During development, granule cell precursors must expand, migrate, and differentiate from the external granule-cell layer to form the internal granule-cell layer, a process that is spatially and temporally regulated by activation of the Hh pathway. Disruption of this process, with constitutive activation of the Hh pathway, can result in the development of medulloblastoma. In mouse models heterozygous for the PTCH1 mutation, medulloblastoma occurs in about one-third of mice by 25 weeks of age. In humans, it has been shown that the Hh pathway is activated, often owing to mutations in PTCH1, SMO, or SUFU, in about 30% of medulloblastomas. There is also preclinical evidence that inhibition of the Hh pathway leads to regression of medulloblastoma tumors in PTCH mutant mice. The first proof of concept for Hh pathway inhibition in medulloblastoma was reported by Rudin and colleagues. They reported a case of a 26-year-old man with refractory medulloblastoma who was treated with daily vismodegib and had a dramatic PR, with rapid regression of tumor burden and reduction of symptoms. The treatment was well tolerated; however, the patient progressed within 3 months of therapy initiation. Molecular analysis of the patient’s tumor pretreatment showed increased expression of Hh target genes and an underlying somatic mutation in PTCH1, suggesting reliance on the Hh pathway. A subsequent analysis of the tumor tissue following progression was undertaken to evaluate the mechanism of treatment resistance. An amino acid substitution at a conserved aspartic acid residue of SMO was identified that had no effect on Hh signaling but disrupted the ability of GDC-0449 to bind SMO and suppress the pathway. This was the first report of a mechanism of resistance to HPIs and will be important in informing future drug development aimed at targeting the Hh pathway. There are currently several ongoing trials investigating HPIs in medulloblastoma in both adults and children. Given the high morbidity associated with treatment of medulloblastoma, as well as the poor outcomes, treatment with HPIs may offer a new therapeutic avenue; however, caution is necessary because the Hh pathway plays such a crucial role in normal development, and, as a result, the effects of HPIs on children will need to be carefully monitored.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree