Gynecologic malignancies carry an estimated incidence of 83,750 cases per year and estimated mortality rate of more than 27,000 women per year. New therapies and therapeutic approaches are needed to improve the outlook for women with gynecologic cancers. Recent insights at the molecular and cellular levels are paving the way for a more directed approach to target mechanisms driving tumorigenesis. This article reviews the roles of new and emerging antiangiogenesis drugs, summarizes the data obtained from clinical trials of antiangiogenic agents, and discusses trials under way to address the role of such strategies in gynecologic cancers.
- •
Recent insights at the molecular and cellular levels are paving the way for a more directed approach to target mechanisms driving tumorigenesis, such as angiogenesis.
- •
Antiangiogenic drugs have shown promise for treatment of gynecologic cancers in phase II and phase III trials.
- •
Clinical responses to antiangiogenesis drugs have been transitory, followed by progressive disease likely due to acquired resistance to such drugs.
Gynecologic malignancies, including cancers of the uterus, ovaries, cervix, fallopian tubes, vagina, and vulva, carry an estimated incidence of 83,750 cases per year and estimated mortality rate of more than 27,000 women per year. Endometrial cancer is the most common gynecologic malignancy. Ovarian cancer, however, remains the most common cause of mortality from a gynecologic cancer. The reason for this is attributed to the advanced stage of ovarian cancer at the time of diagnosis, frequent recurrences, and the emergence of drug resistance. Advances in surgery, chemotherapy, and patient care have improved outcomes for gynecologic malignancies, but overall survival (OS) rates seem to have plateaued. Therefore, new therapies and therapeutic approaches are needed to improve the outlook for women with gynecologic cancers. Recent insights at the molecular and cellular levels are paving the way for a more directed approach to target mechanisms driving tumorigenesis, such as angiogenesis. This article reviews the roles of new and emerging antiangiogenesis drugs, summarizes the data obtained from clinical trials of antiangiogenic agents, and discusses trials under way to address the role of such strategies in gynecologic cancers.
Angiogenesis
Development of new blood supply is essential for the development and maintenance of any tissue or organ. For cancer to grow beyond 1 mm 3 in size, it is necessary for the tumor to develop a sufficient blood supply. Over the past several years, it has become apparent that neovascularization of tumors is a highly complex and regulated process. Classically, there are 2 distinct types of angiogenesis that have been described. The first is sprouting, which involves branching of new blood vessels from pre-existing blood vessels. The second type is splitting or nonsprouting angiogenesis, which involves the splitting of a lumen of an existing vessel. Unlike physiologic angiogenesis, tumor angiogenesis involves endothelial cells that fail to become quiescent. These cells proliferate and grow uncontrollably and have a different phenotype from physiologic vasculature. Morphologically, the tumor vasculature is characterized by irregularly shaped vessels, which are dilated, tortuous, and disorganized.
Recently, other mechanisms of tumor vascularization have been discovered. These include the recruitment of endothelial progenitor cells (EPCs), vessel co-option, vasculogenic mimicry, and lymphangiogenesis. EPCs are circulating cells in the blood that can form new blood vessels. The mobilization and recruitment of EPCs is promoted by several growth factors, chemokines, and cytokines produced during tumor growth. Vessel co-option is a process whereby tumor cells can grow along existing blood vessels without evoking an angiogenic response in such vascular places, such as the brain or lungs. Vasculogenic mimicry is the process of tumor cell plasticity, mainly in aggressive tumors, in which tumor cells dedifferentiate to an endothelial phenotype and make tube-like structures. This mechanism provides an alternate route for tumor vascularization that may be independent of traditional angiogenesis processes. The majority of antiangiogenesis treatments, however, are currently tailored toward the sprouting biology of angiogenesis.
The establishment of angiogenesis relies on several proangiogenic factors, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor, platelet-derived growth factor (PDGF), ephrins, and their receptors. Tumor cells can produce proangiogenic factors for vessel formation. The vessel density and circulating tumor levels of proangiogenic factors VEGF and PDGF are poor prognostic indicators for many solid tumors, including ovarian, endometrial, and cervical carcinomas. Due to their critical role in angiogenesis, proangiogenic factors are attractive therapeutic targets and highly studied in the area of cancer therapeutics.
Bevacizumab
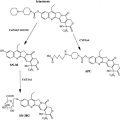
VEGF is a major proangiogenic factor and of the best characterized. It consists of family proteins of which VEGFA (synonymously called VEGF) is the dominant angiogenic factor. It was originally known as vascular permeability factor/VEGF and its mechanism in angiogenesis was unclear. Significant progress in angiogenesis research has elucidated that there are 3 VEGF receptors, with VEGFR-2 most significant for angiogenesis in most solid tumors. On VEGF binding to its receptor on endothelial cells, a cascade of signaling events is activated that results in transcriptional activation of genes responsible for endothelial cell growth. Moreover, activated endothelial cells produce matrix metalloproteinases, which break down the extracellular matrix to allow migration of endothelial cells for new blood vessel formation.
Among the various strategies for targeting VEGF, perhaps the most advanced is the monoclonal antibody bevacizumab. Bevacizumab is a humanized monoclonal antibody directed against human VEGF. It binds to VEGF to block its interaction with VEGF receptors (VEGFR-1 and VEGFR-2), with resultant inhibition of angiogenesis and endothelial cell proliferation. It was the first drug the US Food and Drug Administration (FDA) approved for targeting tumor angiogenesis. Currently, bevacizumab is approved for a variety of solid tumors (eg, colorectal, renal cell, and nonsquamous non–small cell lung cancers and glioblastoma).
Based on encouraging preclinical results, bevacizumab has been investigated clinically in ovarian cancer patients, both in frontline and recurrent disease settings. Response rates among women with recurrent disease ranged from 16% to 24% in initial phase II trials, with median survival of 10.7 to 17 months when bevacizumab was administered either as a single agent or in combination with cyclophosphamide. In a phase II study of recurrent ovarian and primary peritoneal cancer, patients received single-agent bevacizumab every 3 weeks until disease progression or significant toxicity. Of the 62 evaluable patients, 21% had a clinical response, including 2 complete responses. Median progression-free survival (PFS) and OS rates were 4.7 and 17 months, respectively. This regimen was well tolerated, and no association was made between prior platinum sensitivity and hazard to progression or death. In a phase II bevacizumab monotherapy study by Cannistra and colleagues, 44 patients with platinum-resistant epithelial ovarian cancer and peritoneal serous cancer received single-agent bevacizumab every 3 weeks for 5 cycles. Overall response rate was 16%, and median PFS was 4.4 months. This study was terminated early due to a higher than expected incidence of bowel perforation of 11%. More of the toxicities of bevacizumab are discussed later. From these studies, it is apparent that bevacizumab has single-agent activity against ovarian cancer, and subsequent studies addressed its efficacy in combination with cytotoxic agents.
In a phase II study by Penson and colleagues, 62 patients with primary epithelial ovarian, fallopian tube, uterine papillary serous, or primary peritoneal cancer were evaluated using carboplatin and paclitaxel in combination with bevacizumab. All 3 agents were given every 21 days for 6 to 8 cycles followed by bevacizumab every 3 weeks for 1 year. All patients had a CT scan after surgery and before chemotherapy and 45% of the study population had suboptimal cytoreduction (>1 cm residual disease). Radiographic responses were documented in 21 (75%) of 28 women with measurable disease, with CA-125 responses in 76% of patients. The median PFS was 29.8 months. These efficacy results were favorable compared with historical controls. Another phase II study of patients with primary advanced-stage ovarian, peritoneal, or fallopian tube cancer used a treatment protocol of carboplatin/paclitaxel plus bevacizumab for 6 cycles and resulted in an overall 80% response rate. The toxicities were overall well tolerated and no gastrointestinal perforations occurred. Two patients had grade 3 hypertension. A recent phase II, single-institution, open-label trial of intravenous (IV) bevacizumab in combination with intraperitoneal chemotherapy for patients with untreated primary advanced-stage ovarian cancer, however, suggested that bowel toxicity may be exacerbated with this route of administration. Table 1 summarizes selected phase II trials with bevacizumab in gynecologic cancers.
| Study | Therapy | No. of Patients | Selection Criteria | SD (%) | PR (%) | CR (%) | Median PFS (Mo)/Median OS (Mo) |
|---|---|---|---|---|---|---|---|
| Monk et al, 2009 | Bev | 46 | Recur CxCa | NA | 10.9 | 0 | 3.40/7.29 |
| Burger et al, 2007 | Bev | 62 | Recur OvaCa | 51.6 | 17.7 | 3.2 | 4.7/16.9 |
| Cannistra et al, 2007 | Bev | 44 | Ovaca | 61.4 | 15.9 | 0 | 4.4/10.7 |
| Aghajanian et al, 2011 | Bev | 52 | Recur EndoCa | NA | 1.9 | 11.5 | 4.2/10.6 |
| Micha et al, 2007 | CPB/PTX + bev | 20 | OvaCa | 5 | 50 | 30 | NA/NA |
| Penson et al, 2010 | CPB/PTX + bev | 62 | OvaCa | 21 | 55 | 21 | 29.8/NA |
| Garcia et al, 2008 | Cyclo + bev | 70 | Recur OvaCa | 63 | 24 | 0 | 7.2/16.9 |
| Konner et al, 2011 | IV/IP PTX + IP CDDP + bev | 41 | OvaCa | NA | NA | NA | 28.6/NA |
There are several phase III clinical trials under way or recently completed in ovarian cancer. The Gynecologic Oncology Group (GOG) 218 and International Collaboration on Ovarian Neoplasms (ICON) 7 are 2 randomized phase III studies that include combination chemotherapy with maintenance therapy. In GOG 218, 1873 women with previously untreated advanced epithelial ovarian, primary peritoneal, or fallopian tube carcinoma showed that women who received bevacizumab in combination with paclitaxel and carboplatin, and continued on bevacizumab maintenance therapy for a total duration of 15 months, had a median PFS of 14.1 months compared with 10.3 months in women who received chemotherapy alone (hazard ratio [HR] 0.72, P <.0001). ICON7 included 1528 women with previously untreated epithelial ovarian, primary peritoneal, or fallopian tube carcinoma. Women who received bevacizumab in combination with paclitaxel and carboplatin and continued use of bevacizumab maintenance for a total duration of up to 12 months had a median PFS of 18.3 months compared with 16 months in women who received chemotherapy alone (HR 0.79, P = .001).
Several studies were also launched in the setting of relapsed ovarian cancer. The GOG 213 (NCT00565851) and OCEANS (NCT00434642) studies are evaluating chemotherapy and bevacizumab combinations (paclitaxel/carboplatin and gemcitabine/carboplatin, respectively) in patients with recurrent platinum-sensitive disease. The OCEANS study recently reported safety and efficacy data in 484 patients stratified by length of platinum-free interval and performance of secondary surgery. Unique in this design was the ability to maintain bevacizumab therapy to progression after 6 to 10 cycles of concomitant therapy with gemcitabine and carboplatin. No gastrointestinal perforations were observed on either arm of this placebo-controlled trial. Grade 3 hypertension and proteinuria were more frequently observed in the bevacizumab arm.The median PFS of the experimental arm was 12.4 months, however, and compared favorably with 8.4 months in the control arm (HR 0.484; 95% CI, 0.39–0.61; P <.0001). OS was immature at this report. The AURELIA trial is evaluating the addition of bevacizumab to paclitaxel, topotecan, and liposomal doxorubicin in patients with platinum-resistant ovarian cancer. Table 2 shows the completed, ongoing, and future phase III trials of bevacizumab in gynecologic cancers.
| Trial | Site of Disease | Drug Regimens | Date |
|---|---|---|---|
| GOG 218 | OvaCa | CPB + PTX vs CBP + PTX + bev vs CBP + PTX + bev, then maintenance bev | Sep 2005 to Oct 2008 |
| ICON7 | OvaCa | CBP + PTX with and without bev, then maintenance bev | Opened Apr 2006 |
| GOG 252 | OvaCa | IV vs IP platinum + PTX with IV bev, then maintenance bev | Opened Aug 2009 |
| GOG 262 | OvaCa | Dose dense PTX with bev | Opened Feb 2010 |
| GOG 213 | Platinum-sensitive recur OvaCa | CBP + PTX with and without bev, then maintenance bev | Opened Dec 2007 |
| OCEANS | Platinum-sensitive recur OvaCa | CBP + GCB with and without bev | 2007–2011 |
| AURELIA | Platinum-resistant OvaCa | PTX + TPT + LD with and without bev | Opened Oct 2009 |
| GCIG | Stage II–IV or recur MucOvaCa | CBP + PTX with and without bev, then maintenance bev vs OX + CAP with and without bev, then maintenance bev | Opened Jan 2010 |
| GOG 240 | Stage IVB, recur CxCa | CDDP + PTX with and without bev vs TPT/PTX with and without bev | Opened Apr 2009 |
Phase II studies have also reported some response with use of bevacizumab alone in the setting of persistent or recurrent endometrial and cervical cancer. In patients with recurrent endometrial cancer, bevacizumab treatment resulted in a response rate of 13.5% (1 complete response and 7 partial responses) with a median PFS of 3.4 months and OS of 7.29 months. In patients with persistent or recurrent squamous cell carcinoma of the cervix, 23.9% had progression-free interval disease for 6 months and 10.9% had a partial response. The median PFS was 3.40 months and OS of 7.29 months. This compared favorably with historical GOG phase II trials in this setting. Table 1 provides a summary of selected phase II trials with bevacizumab in gynecologic cancers.
Although anti-VEGF treatments show some promise, there are concerns related to toxicity. The toxicities associated with bevacizumab have been documented from various trials and include hypertension, proteinuria, hemorrhage, neutropenia, venous thromboembolism, pulmonary embolus, congestive heart failure, myocardial infarction, and cerebrovascular ischemia. Hypertension is one the most common side effects of bevacizumab. The pathogenesis of bevacizumab-induced hypertension is not thoroughly understood. It is thought that VEGF antagonism can cause a decrease in nitric-oxide production by inhibition of nitric oxide synthase. Suppression of nitric oxide leads to vasoconstriction and decreased sodium ion renal excretion leading to high blood pressure. The occurrence of hypertension is dose dependent. For example, the overall incidence of hypertension in patients receiving low-dose (3, 5, or 7.5 mg/kg/dose) versus higher-dose (10 or 15 mg/kg/dose) single-agent bevacizumab is 2.7% to 32% and 17.6% to 36%, respectively. This bevacizumab toxicity may be useful as a clinical response parameter in patients. Among breast non–small cell lung cancer patients or colorectal cancer patients treated with bevacizumab, those with grades 2–4 hypertension had longer median survival compared with those without such elevation in blood pressure. Perren and colleagues and Scartozzi and colleagues showed that in metastatic colorectal patients treated with bevacizumab, the median PFS was 14.5 months for patients showing bevacizumab-related hypertension, whereas it was 3.1 months in those without hypertension ( P = .04). Although hypertension may be a good clinical measure of treatment response, bevacizumab-induced hypertension must be treated to avoid cardiovascular injury. Furthermore, permanent discontinuation of bevacizumab is recommended in patients who have hypertensive crisis.
Proteinuria in response to bevacizumab can occur as a result of interference with VEGF-dependent glomerular endothelial injury. It can also occur due to thrombotic microangiopathy. The proteinuria is typically asymptomatic and detected incidentally. Monitoring by use of regular urine dipstick should be considered. Those with dipstick reading of 2 g or more should undergo 24-hour urine total protein collection. Bevacizumab should be stopped if a patient is excreting at least 2 g of protein in a 24-hour period. Treatment may resume if the patient recovers within 3 weeks and has no sign of nephrotic syndrome.
There is evidence of increased risk of arterial thromboembolic events associated with bevacizumab therapy. In a pooled data analysis of 1745 patient with metastatic colorectal cancer, non–small cell lung cancer, or breast cancer from 5 randomized trials, the addition of bevacizumab to chemotherapy was associated with increased risk of arterial thromboembolic events (overall incidence was 3.8% with bevacizumab vs 1.7% with chemotherapy). There was no difference with regard to venous thromboembolic events between the 2 groups.
One of the most worrisome complications of bevacizumab in the setting of gynecologic cancers is intestinal perforation. Two phase II trials of bevacizumab in the treatment of ovarian cancer were stopped early due to a high rate of intestinal perforation (11%–15%). Other studies have shown smaller incidence of intestinal perforation of approximately 4% to 5% in ovarian cancer. Perforations are thought more prevalent in those with acute diverticulitis, intra-abdominal abscess, gastrointestinal obstruction, tumor at perforation site, abdominal carcinomatosis, and previous abdominal or pelvic radiotherapy. Therefore, careful patient selection to reduce risk should be considered by limiting or excluding bevacizumab treatment in patients with clinical symptoms of bowel obstruction, rectosigmoid involvement on examination physical examination, and bowel involvement on CT. There is an increased risk of wound healing complications in patients receiving bevacizumab. It is recommended that there is a 30-day window between discontinuation of bevacizumab and major surgery to lower the risk of surgical wound or bowel anastomosis complications.
Bevacizumab
VEGF is a major proangiogenic factor and of the best characterized. It consists of family proteins of which VEGFA (synonymously called VEGF) is the dominant angiogenic factor. It was originally known as vascular permeability factor/VEGF and its mechanism in angiogenesis was unclear. Significant progress in angiogenesis research has elucidated that there are 3 VEGF receptors, with VEGFR-2 most significant for angiogenesis in most solid tumors. On VEGF binding to its receptor on endothelial cells, a cascade of signaling events is activated that results in transcriptional activation of genes responsible for endothelial cell growth. Moreover, activated endothelial cells produce matrix metalloproteinases, which break down the extracellular matrix to allow migration of endothelial cells for new blood vessel formation.
Among the various strategies for targeting VEGF, perhaps the most advanced is the monoclonal antibody bevacizumab. Bevacizumab is a humanized monoclonal antibody directed against human VEGF. It binds to VEGF to block its interaction with VEGF receptors (VEGFR-1 and VEGFR-2), with resultant inhibition of angiogenesis and endothelial cell proliferation. It was the first drug the US Food and Drug Administration (FDA) approved for targeting tumor angiogenesis. Currently, bevacizumab is approved for a variety of solid tumors (eg, colorectal, renal cell, and nonsquamous non–small cell lung cancers and glioblastoma).
Based on encouraging preclinical results, bevacizumab has been investigated clinically in ovarian cancer patients, both in frontline and recurrent disease settings. Response rates among women with recurrent disease ranged from 16% to 24% in initial phase II trials, with median survival of 10.7 to 17 months when bevacizumab was administered either as a single agent or in combination with cyclophosphamide. In a phase II study of recurrent ovarian and primary peritoneal cancer, patients received single-agent bevacizumab every 3 weeks until disease progression or significant toxicity. Of the 62 evaluable patients, 21% had a clinical response, including 2 complete responses. Median progression-free survival (PFS) and OS rates were 4.7 and 17 months, respectively. This regimen was well tolerated, and no association was made between prior platinum sensitivity and hazard to progression or death. In a phase II bevacizumab monotherapy study by Cannistra and colleagues, 44 patients with platinum-resistant epithelial ovarian cancer and peritoneal serous cancer received single-agent bevacizumab every 3 weeks for 5 cycles. Overall response rate was 16%, and median PFS was 4.4 months. This study was terminated early due to a higher than expected incidence of bowel perforation of 11%. More of the toxicities of bevacizumab are discussed later. From these studies, it is apparent that bevacizumab has single-agent activity against ovarian cancer, and subsequent studies addressed its efficacy in combination with cytotoxic agents.
In a phase II study by Penson and colleagues, 62 patients with primary epithelial ovarian, fallopian tube, uterine papillary serous, or primary peritoneal cancer were evaluated using carboplatin and paclitaxel in combination with bevacizumab. All 3 agents were given every 21 days for 6 to 8 cycles followed by bevacizumab every 3 weeks for 1 year. All patients had a CT scan after surgery and before chemotherapy and 45% of the study population had suboptimal cytoreduction (>1 cm residual disease). Radiographic responses were documented in 21 (75%) of 28 women with measurable disease, with CA-125 responses in 76% of patients. The median PFS was 29.8 months. These efficacy results were favorable compared with historical controls. Another phase II study of patients with primary advanced-stage ovarian, peritoneal, or fallopian tube cancer used a treatment protocol of carboplatin/paclitaxel plus bevacizumab for 6 cycles and resulted in an overall 80% response rate. The toxicities were overall well tolerated and no gastrointestinal perforations occurred. Two patients had grade 3 hypertension. A recent phase II, single-institution, open-label trial of intravenous (IV) bevacizumab in combination with intraperitoneal chemotherapy for patients with untreated primary advanced-stage ovarian cancer, however, suggested that bowel toxicity may be exacerbated with this route of administration. Table 1 summarizes selected phase II trials with bevacizumab in gynecologic cancers.
| Study | Therapy | No. of Patients | Selection Criteria | SD (%) | PR (%) | CR (%) | Median PFS (Mo)/Median OS (Mo) |
|---|---|---|---|---|---|---|---|
| Monk et al, 2009 | Bev | 46 | Recur CxCa | NA | 10.9 | 0 | 3.40/7.29 |
| Burger et al, 2007 | Bev | 62 | Recur OvaCa | 51.6 | 17.7 | 3.2 | 4.7/16.9 |
| Cannistra et al, 2007 | Bev | 44 | Ovaca | 61.4 | 15.9 | 0 | 4.4/10.7 |
| Aghajanian et al, 2011 | Bev | 52 | Recur EndoCa | NA | 1.9 | 11.5 | 4.2/10.6 |
| Micha et al, 2007 | CPB/PTX + bev | 20 | OvaCa | 5 | 50 | 30 | NA/NA |
| Penson et al, 2010 | CPB/PTX + bev | 62 | OvaCa | 21 | 55 | 21 | 29.8/NA |
| Garcia et al, 2008 | Cyclo + bev | 70 | Recur OvaCa | 63 | 24 | 0 | 7.2/16.9 |
| Konner et al, 2011 | IV/IP PTX + IP CDDP + bev | 41 | OvaCa | NA | NA | NA | 28.6/NA |
There are several phase III clinical trials under way or recently completed in ovarian cancer. The Gynecologic Oncology Group (GOG) 218 and International Collaboration on Ovarian Neoplasms (ICON) 7 are 2 randomized phase III studies that include combination chemotherapy with maintenance therapy. In GOG 218, 1873 women with previously untreated advanced epithelial ovarian, primary peritoneal, or fallopian tube carcinoma showed that women who received bevacizumab in combination with paclitaxel and carboplatin, and continued on bevacizumab maintenance therapy for a total duration of 15 months, had a median PFS of 14.1 months compared with 10.3 months in women who received chemotherapy alone (hazard ratio [HR] 0.72, P <.0001). ICON7 included 1528 women with previously untreated epithelial ovarian, primary peritoneal, or fallopian tube carcinoma. Women who received bevacizumab in combination with paclitaxel and carboplatin and continued use of bevacizumab maintenance for a total duration of up to 12 months had a median PFS of 18.3 months compared with 16 months in women who received chemotherapy alone (HR 0.79, P = .001).
Several studies were also launched in the setting of relapsed ovarian cancer. The GOG 213 (NCT00565851) and OCEANS (NCT00434642) studies are evaluating chemotherapy and bevacizumab combinations (paclitaxel/carboplatin and gemcitabine/carboplatin, respectively) in patients with recurrent platinum-sensitive disease. The OCEANS study recently reported safety and efficacy data in 484 patients stratified by length of platinum-free interval and performance of secondary surgery. Unique in this design was the ability to maintain bevacizumab therapy to progression after 6 to 10 cycles of concomitant therapy with gemcitabine and carboplatin. No gastrointestinal perforations were observed on either arm of this placebo-controlled trial. Grade 3 hypertension and proteinuria were more frequently observed in the bevacizumab arm.The median PFS of the experimental arm was 12.4 months, however, and compared favorably with 8.4 months in the control arm (HR 0.484; 95% CI, 0.39–0.61; P <.0001). OS was immature at this report. The AURELIA trial is evaluating the addition of bevacizumab to paclitaxel, topotecan, and liposomal doxorubicin in patients with platinum-resistant ovarian cancer. Table 2 shows the completed, ongoing, and future phase III trials of bevacizumab in gynecologic cancers.
| Trial | Site of Disease | Drug Regimens | Date |
|---|---|---|---|
| GOG 218 | OvaCa | CPB + PTX vs CBP + PTX + bev vs CBP + PTX + bev, then maintenance bev | Sep 2005 to Oct 2008 |
| ICON7 | OvaCa | CBP + PTX with and without bev, then maintenance bev | Opened Apr 2006 |
| GOG 252 | OvaCa | IV vs IP platinum + PTX with IV bev, then maintenance bev | Opened Aug 2009 |
| GOG 262 | OvaCa | Dose dense PTX with bev | Opened Feb 2010 |
| GOG 213 | Platinum-sensitive recur OvaCa | CBP + PTX with and without bev, then maintenance bev | Opened Dec 2007 |
| OCEANS | Platinum-sensitive recur OvaCa | CBP + GCB with and without bev | 2007–2011 |
| AURELIA | Platinum-resistant OvaCa | PTX + TPT + LD with and without bev | Opened Oct 2009 |
| GCIG | Stage II–IV or recur MucOvaCa | CBP + PTX with and without bev, then maintenance bev vs OX + CAP with and without bev, then maintenance bev | Opened Jan 2010 |
| GOG 240 | Stage IVB, recur CxCa | CDDP + PTX with and without bev vs TPT/PTX with and without bev | Opened Apr 2009 |
Phase II studies have also reported some response with use of bevacizumab alone in the setting of persistent or recurrent endometrial and cervical cancer. In patients with recurrent endometrial cancer, bevacizumab treatment resulted in a response rate of 13.5% (1 complete response and 7 partial responses) with a median PFS of 3.4 months and OS of 7.29 months. In patients with persistent or recurrent squamous cell carcinoma of the cervix, 23.9% had progression-free interval disease for 6 months and 10.9% had a partial response. The median PFS was 3.40 months and OS of 7.29 months. This compared favorably with historical GOG phase II trials in this setting. Table 1 provides a summary of selected phase II trials with bevacizumab in gynecologic cancers.
Although anti-VEGF treatments show some promise, there are concerns related to toxicity. The toxicities associated with bevacizumab have been documented from various trials and include hypertension, proteinuria, hemorrhage, neutropenia, venous thromboembolism, pulmonary embolus, congestive heart failure, myocardial infarction, and cerebrovascular ischemia. Hypertension is one the most common side effects of bevacizumab. The pathogenesis of bevacizumab-induced hypertension is not thoroughly understood. It is thought that VEGF antagonism can cause a decrease in nitric-oxide production by inhibition of nitric oxide synthase. Suppression of nitric oxide leads to vasoconstriction and decreased sodium ion renal excretion leading to high blood pressure. The occurrence of hypertension is dose dependent. For example, the overall incidence of hypertension in patients receiving low-dose (3, 5, or 7.5 mg/kg/dose) versus higher-dose (10 or 15 mg/kg/dose) single-agent bevacizumab is 2.7% to 32% and 17.6% to 36%, respectively. This bevacizumab toxicity may be useful as a clinical response parameter in patients. Among breast non–small cell lung cancer patients or colorectal cancer patients treated with bevacizumab, those with grades 2–4 hypertension had longer median survival compared with those without such elevation in blood pressure. Perren and colleagues and Scartozzi and colleagues showed that in metastatic colorectal patients treated with bevacizumab, the median PFS was 14.5 months for patients showing bevacizumab-related hypertension, whereas it was 3.1 months in those without hypertension ( P = .04). Although hypertension may be a good clinical measure of treatment response, bevacizumab-induced hypertension must be treated to avoid cardiovascular injury. Furthermore, permanent discontinuation of bevacizumab is recommended in patients who have hypertensive crisis.
Proteinuria in response to bevacizumab can occur as a result of interference with VEGF-dependent glomerular endothelial injury. It can also occur due to thrombotic microangiopathy. The proteinuria is typically asymptomatic and detected incidentally. Monitoring by use of regular urine dipstick should be considered. Those with dipstick reading of 2 g or more should undergo 24-hour urine total protein collection. Bevacizumab should be stopped if a patient is excreting at least 2 g of protein in a 24-hour period. Treatment may resume if the patient recovers within 3 weeks and has no sign of nephrotic syndrome.
There is evidence of increased risk of arterial thromboembolic events associated with bevacizumab therapy. In a pooled data analysis of 1745 patient with metastatic colorectal cancer, non–small cell lung cancer, or breast cancer from 5 randomized trials, the addition of bevacizumab to chemotherapy was associated with increased risk of arterial thromboembolic events (overall incidence was 3.8% with bevacizumab vs 1.7% with chemotherapy). There was no difference with regard to venous thromboembolic events between the 2 groups.
One of the most worrisome complications of bevacizumab in the setting of gynecologic cancers is intestinal perforation. Two phase II trials of bevacizumab in the treatment of ovarian cancer were stopped early due to a high rate of intestinal perforation (11%–15%). Other studies have shown smaller incidence of intestinal perforation of approximately 4% to 5% in ovarian cancer. Perforations are thought more prevalent in those with acute diverticulitis, intra-abdominal abscess, gastrointestinal obstruction, tumor at perforation site, abdominal carcinomatosis, and previous abdominal or pelvic radiotherapy. Therefore, careful patient selection to reduce risk should be considered by limiting or excluding bevacizumab treatment in patients with clinical symptoms of bowel obstruction, rectosigmoid involvement on examination physical examination, and bowel involvement on CT. There is an increased risk of wound healing complications in patients receiving bevacizumab. It is recommended that there is a 30-day window between discontinuation of bevacizumab and major surgery to lower the risk of surgical wound or bowel anastomosis complications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree