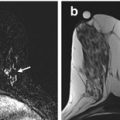
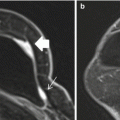
Fig. 11.1
Posteriorly located lesion that is not amenable for MRI-guided biopsy. (a) Subtracted sagittal T1W post-contrast image. (b) MRI-directed US from right breast. A 27-year-old woman with BRCA2 gene mutation found to have an oval enhancing mass (circle in a) in the right breast in the far posterior aspect, just anterior to the pectoralis major muscle. MRI-directed US identified a 9-mm oval hypoechoic mass at 10:00, 5-cm from the nipple (circle in b). US-guided FNA aspiration was performed demonstrating fibroadenoma, which is benign and concordant. This mass remained stable at 12-months follow-up
11.2 Technique in Performing MRI-Directed US
Thorough and careful review of the breast MRI is essential prior to performing an MRI-directed ultrasound. If a technologist performs the ultrasound study, it is also important to review the MRI study with the technologist. When reviewing the breast MRI study, utilization of 3D reconstructions can help make it easier to understand the location of the lesion in all 3 planes (see Fig. 11.2) and its relationship to surrounding structures [24]. The location and morphology of the MRI-detected lesion are important information to know to determine the expected location and appearance of the lesion at ultrasound.
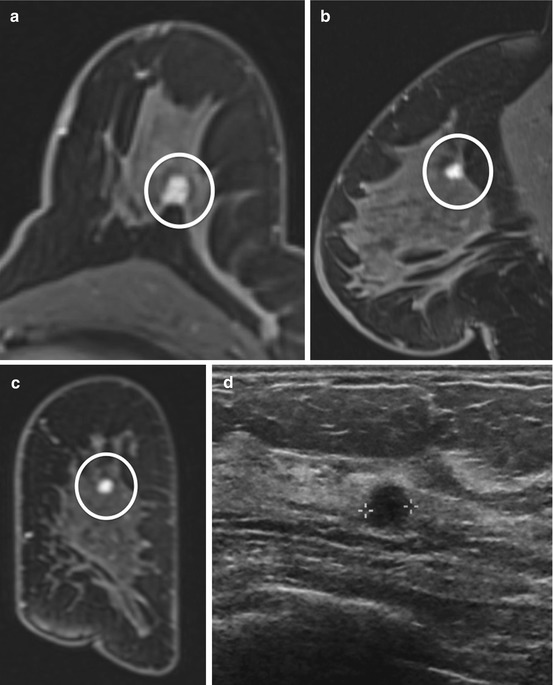

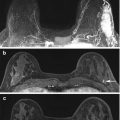
Fig. 11.2
3D reconstructions can help make it easier to understand the location of the lesion in all 3 planes. (a) Axial T1W post-contrast image. (b) Sagittal T1W post-contrast image. (c) Reconstructed coronal post-contrast image. (d) MRI-direct US from left breast. 39 year-old found on extent of disease MRI to have an enhancing round mass with irregular margin (circle) in the left breast at 12:00, 5-cm from the nipple. The 3 planes aided in ultrasound localization of the mass (calipers). Biopsy demonstrated invasive ductal carcinoma
Lesion location information to note includes the quadrant and o’clock position, distance from nipple, skin, and chest wall, anatomic relationship to surrounding tissue, and its relationship to other landmarks. It is important to keep in mind that the positioning of the breast is different between MRI and ultrasound [19, 24]. Breast US is performed with the patient in the supine or supine oblique position with the arm raised while breast MRI is performed with the patient in the prone position. In the supine position with the arm raised, the breast tissue is flattened and widened which makes the breast tissue, including breast lesions, appear more compact. The distance between the chest wall and the glandular tissue is decreased on US relative to MRI (see Fig. 11.3). With MRI, the breast in the prone position is pendant with little to no compression; this results in the tissue appearing more stretched in the anterior to posterior dimension (see Fig. 11.3). The distance between the chest wall and the glandular tissue is increased and lesions can appear more anterior on MRI than on US images [19, 24]. Carbonaro et al. showed lesion displacement of about 3–6 cm along the three orthogonal directions on prone versus supine MRI [4]. The o’clock position of the lesion in ultrasound can also vary by one or two hours compared to the MRI [17]. Since lesion displacement can vary between ultrasound and MRI, the anatomic relationship of the lesion to surround tissue (subcutaneous fat, glandular tissue, or retroglandular fat) (see Fig. 11.3) can be used to help in identifying a correlate with more confidence [19]. The relationship of the lesion to surrounding tissue is maintained between the two modalities.
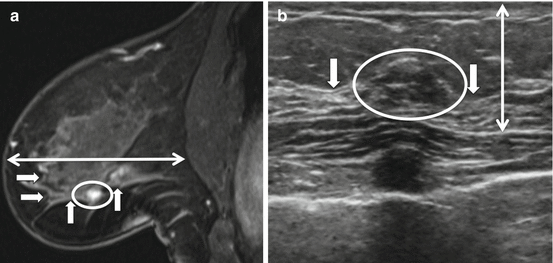

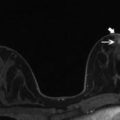
Fig. 11.3
Effects of the breast in the prone position at MRI and supine position at US and the relationship of lesion to surrounding tissue. (a) Subtracted sagittal T1W post-contrast image with patient in prone position. (b) MRI-directed US image from left breast. Breast tissue in the prone position appears more stretched in the anterior to posterior dimension (double arrowhead in a) while in the supine position along with compression by the ultrasound probe, the breast tissue becomes flattened and widened. In the supine position, the breast tissue, including breast lesions, appear more compact (double arrowhead in b). 41-year-old woman with a strong family history of breast cancer was found to have an oval irregular enhancing mass (circle in a) in the left breast middle depth which at ultrasound, the mass (circle in b) appears more posteriorly located due to flattening of the breast tissue. However, the lesion’s relationship to surrounding tissue is maintained between the two modalities (glandular tissue indicted by arrows). Biopsy demonstrated fibroadenoma
More reliable location information to note is the distance to the skin and nipple (see Fig. 11.4) as suggested by Carbonaro et al. [4]. The median lesion-to-skin and lesion-to-nipple displacements were less than 1 cm and that the lesion-to-nipple distance may be the most reliable measure to be used for MRI-directed US [4]. In addition to using the skin and nipple as fixed markers, the relationship of the lesion to co-existing lesions such as cysts, scars, implants, clips, known cancer (see Fig. 11.5), or known fibroadenomas may be helpful. Knowledge of co-existing lesions is also important to prevent erroneous correlation, particularly in patients with multiple lesions within a similar region of the breast [19].
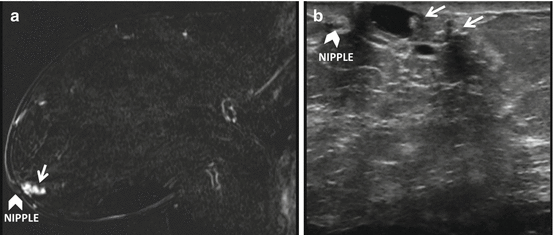
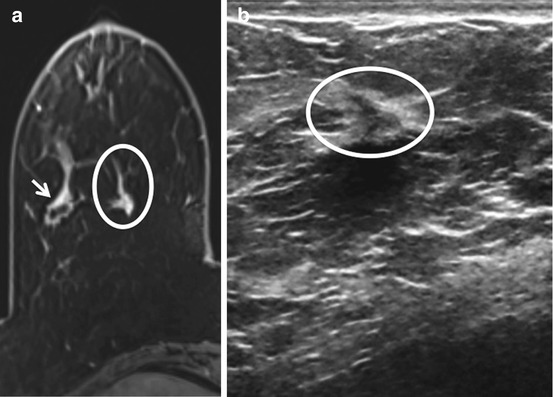

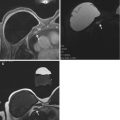
Fig. 11.4
Nipple as a fixed landmark. (a) Subtracted T1W post-contrast image. (b) MRI-directed US from left breast. Sixty-eight year-old with history of breast cancer found on surveillance MRI to have an oval mass with irregular margins (arrow) in the left retroareolar breast subjacent to the nipple (arrowhead). Using the nipple (arrowhead) as a fixed landmark, an irregular hypoechoic mass was identified within a focally dilated duct at US. Biopsy yielded papillary lesion

Fig. 11.5
Known cancer as a landmark. (a) Axial T1W post-contrast image. (b) MRI-directed US from right breast. Sixty-four-year-old woman with known right breast invasive ductal carcinoma (arrow) found on extent of disease MRI to have an irregular enhancing mass (circle a) medial to the known malignancy. Using the known malignancy as a landmark, a subtle sonographic correlate (circle b) was identified. Biopsy demonstrated a second area of invasive ductal carcinoma.
MRI lesion morphology with respect to shape, size and contours can also be useful in finding a lesion on MRI-directed ultrasound (see Fig. 11.6). Perfect morphologic agreement of lesions between the two modalities must not necessarily be expected [10]. Lesions at US tend to look smaller than at MRI as they are compressed in a vertical direction by the ultrasound probe. In addition, round lesions at MRI often appear oval or elliptical at ultrasound [19].
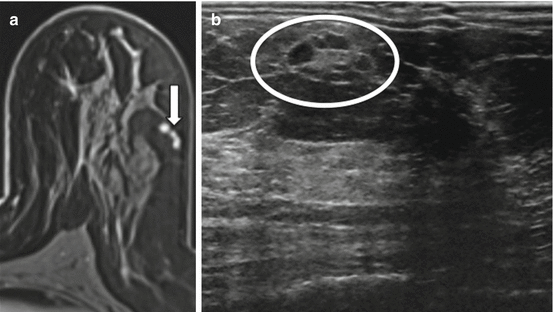

Fig. 11.6
Shape, size and contours can be useful in finding a lesion on MRI-directed ultrasound. (a) Axial T1W post-contrast image. (b) MRI-directed US from left breast. Forty-seven year-old woman with known malignancy was found on extent of disease MRI to have several enhancing contiguous masses (arrow) in the left breast at 2:00, 5 cm from the nipple. At ultrasound, several oval, circumscribed adjacent hypoechoic masses (circle) were identified similar in shape, size, and contour to the MRI lesion. At biopsy, the masses represented the lobulated cortex of a benign lymph node
11.3 Evidence-Based Findings
11.3.1 Frequency of Sonographic Correlate for MRI-Detected Lesions
Several studies have investigated the frequency at which MRI-directed ultrasound identifies a sonographic correlate for a lesion initially detected on MRI. These studies vary widely in rates of correlate, most likely because of heterogeneous methodologies and study populations, and also the inherently user-dependent nature of ultrasound [12, 22]. Limitations of the studies generally included retrospective design and lack of defined protocol establishing which lesions underwent MRI-directed ultrasound versus MRI-guided biopsy directly [5, 12, 13, 22]. In 2014 Spick and Baltzer published a meta-analysis of 17 studies that found a pooled detection rate for sonographic correlate of 58 %, with a wide reported range of 22–82 % [22]. Analyses of lesion characteristics have helped to understand which given MRI lesions are the most likely to have ultrasound correlates, with most studies showing masses and malignant lesions to be the most likely MRI-detected findings to also be seen on ultrasound.
11.3.2 Lesion Type
The three primary enhancing lesion types as defined by the BI-RADS lexicon [16], mass, focus and non-mass enhancement, show varying rates of sonographic correlate. Masses have been shown by many studies to be the lesion type most likely to have a correlate. In their meta-analysis, Spick and Baltzer found that mass lesions were more likely than non-mass enhancement to have a correlate (p < .0001) [22]. Many single studies have also demonstrated statistical significance for MRI-detected masses having a higher rate of sonographic correlate than non-mass enhancement. Meissnitzer et al. found a sonographic correlate for 62 % of masses and 31 % of non-mass enhancement (p < 0.001) [13]. Abe et al. found a correlate for 67 % of MRI-detected masses and 12 % of non-mass enhancement (p < 0.005) [1]; these authors also reported a 46 % correlate rate for foci, an intermediate rate between that of the other two lesion types [1]. Similarly, Hollowell reported a correlate rate of 49 % for masses, 42 % for foci, and 15 % for non-mass enhancement (p = .0006) [7]. DeMartini et al. found MRI-directed ultrasound yield to be higher for masses (58 %) than for foci (37 %) or non-mass enhancement (30 %) [5].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree