Although selective mutant BRAF inhibitors have revolutionized the treatment of metastatic melanoma, the magnitude and duration of their clinical benefit are significantly undermined by de novo and acquired resistance. Functional studies, molecular characterization of clinical samples, and clinical trials are providing insights into the landscape of resistance mechanisms in this disease. These findings have implications for the development of rational therapeutic approaches, and have identified several challenges that remain to be overcome if outcomes are to be improved in patients with metastatic melanoma.
Key points
- •
The clinical benefit of BRAF and MEK inhibitors is limited by both de novo and acquired resistance mechanisms.
- •
The presence of molecular changes that cause reactivation of the MAPK pathway is the most common feature of tumors that have progressed on BRAF-inhibitor therapy.
- •
Activation of the PI3K/AKT pathway is commonly seen concurrently with reactivation of MAPK pathway signaling in BRAF inhibitor–resistant tumors.
- •
Resistance may be mediated by genetic and epigenetic events, and there is growing evidence of intrapatient and intratumoral heterogeneity of resistance mechanisms.
- •
Inhibiting multiple pathways simultaneously may be required to overcome resistance to MAPK pathway inhibitors.
Introduction
The era of personalized therapy in melanoma launched with the identification of recurrent BRAF V600 mutations in more than 50% of melanomas. Valine-600 is a key residue in the activation segment of the kinase domain of the BRAF protein, and mutation of this residue to glutamate (V600E) increases the protein’s catalytic activity by 500 fold or more. Expression of the BRAF V600 oncoprotein constitutively activates the mitogen-activated protein kinase (MAPK) signaling pathway, thereby promoting proliferation and survival. Although initial clinical trials with nonselective BRAF inhibitors (ie, sorafenib) yielded disappointing results, 2 different small molecules (vemurafenib and dabrafenib) that selectively inhibit the V600-mutant form of BRAF demonstrated unprecedented single-agent activity in early-phase clinical trials. Both agents went on to demonstrate significant improvements in objective response rates (ORR) (∼50%) and progression-free survival (PFS) (median 5–6 months) in phase III randomized clinical trials versus chemotherapy, leading to their regulatory approval by the US Food and Drug Administration (FDA) for BRAF V600 – positive metastatic melanoma.
The rapid progress from the identification of this driver mutation to FDA-approved therapies stands as a prime example of the potential benefit of translational research and personalized cancer therapy. However, although the development of the BRAFi was a breakthrough in this highly aggressive disease, the benefit of these agents in patients is critically limited by resistance. Multiple clinical studies have demonstrated that even in patients with the same activating BRAF mutation, the degree of tumor shrinkage achieved can be markedly heterogeneous, with approximately 10% of patients failing to achieve any reduction in tumor burden. These observations reflect the presence of de novo, or preexisting, resistance mechanisms that limit the efficacy of the BRAFi. Although most patients achieve some degree of tumor shrinkage, approximately 90% demonstrate disease progression within 12 months of starting treatment. This renewed growth is due to the acquisition of molecular changes that mediate resistance (ie, acquired resistance).
Numerous studies have been undertaken to identify the predictors and mechanisms of resistance to mutant-selective BRAFi. These studies have identified alterations in several key signaling molecules and pathways that mediate resistance, and have presented new therapeutic targets for combinatorial strategies for melanomas with BRAF V600 mutations ( Table 1 ). Furthermore, these studies have generated numerous insights that will inform and facilitate the development of new treatments for other candidate targets in this disease.
| Pathway | Type of Aberration | Aberration | Type of Resistance | Prevalence in Resistant Tumors (%) | References |
|---|---|---|---|---|---|
| MAPK | Genetic | Activating mutations in NRAS | Acquired | 18–23 | |
| Genetic | Activating mutations in KRAS | Acquired | 7 | ||
| Genetic | BRAF amplification | Acquired | 9–19 | ||
| Genetic | Activating mutations in MAP2K1 | Acquired | 4–7 | ||
| Genetic | Activating mutations in MAP2K2 | De novo and acquired | 9 | ||
| Genetic | Loss-of-function mutations in NF1 | De novo and acquired | 2 | ||
| Epigenetic | BRAF splicing | Acquired | 14–31 | ||
| PI3K/AKT | Genetic or epigenetic | Inactivating mutation, deletion, or loss of expression in PTEN | De novo and acquired | ∼9 | |
| Genetic | Activating mutations in PIK3CA or PIK3CG | Acquired | 4–6 | ||
| Genetic | Inactivating mutations in PIK3R2 | Acquired | 2 | ||
| Genetic | Activating mutations in AKT1 or AKT3 | Acquired | 4 | ||
| Others | Epigenetic | RTK overexpression or activation | Acquired | Not available | |
| Genetic | CDKN2A copy loss | De novo and acquired | 7 | ||
| Genetic | MITF amplification | Acquired | 2 |
Introduction
The era of personalized therapy in melanoma launched with the identification of recurrent BRAF V600 mutations in more than 50% of melanomas. Valine-600 is a key residue in the activation segment of the kinase domain of the BRAF protein, and mutation of this residue to glutamate (V600E) increases the protein’s catalytic activity by 500 fold or more. Expression of the BRAF V600 oncoprotein constitutively activates the mitogen-activated protein kinase (MAPK) signaling pathway, thereby promoting proliferation and survival. Although initial clinical trials with nonselective BRAF inhibitors (ie, sorafenib) yielded disappointing results, 2 different small molecules (vemurafenib and dabrafenib) that selectively inhibit the V600-mutant form of BRAF demonstrated unprecedented single-agent activity in early-phase clinical trials. Both agents went on to demonstrate significant improvements in objective response rates (ORR) (∼50%) and progression-free survival (PFS) (median 5–6 months) in phase III randomized clinical trials versus chemotherapy, leading to their regulatory approval by the US Food and Drug Administration (FDA) for BRAF V600 – positive metastatic melanoma.
The rapid progress from the identification of this driver mutation to FDA-approved therapies stands as a prime example of the potential benefit of translational research and personalized cancer therapy. However, although the development of the BRAFi was a breakthrough in this highly aggressive disease, the benefit of these agents in patients is critically limited by resistance. Multiple clinical studies have demonstrated that even in patients with the same activating BRAF mutation, the degree of tumor shrinkage achieved can be markedly heterogeneous, with approximately 10% of patients failing to achieve any reduction in tumor burden. These observations reflect the presence of de novo, or preexisting, resistance mechanisms that limit the efficacy of the BRAFi. Although most patients achieve some degree of tumor shrinkage, approximately 90% demonstrate disease progression within 12 months of starting treatment. This renewed growth is due to the acquisition of molecular changes that mediate resistance (ie, acquired resistance).
Numerous studies have been undertaken to identify the predictors and mechanisms of resistance to mutant-selective BRAFi. These studies have identified alterations in several key signaling molecules and pathways that mediate resistance, and have presented new therapeutic targets for combinatorial strategies for melanomas with BRAF V600 mutations ( Table 1 ). Furthermore, these studies have generated numerous insights that will inform and facilitate the development of new treatments for other candidate targets in this disease.
| Pathway | Type of Aberration | Aberration | Type of Resistance | Prevalence in Resistant Tumors (%) | References |
|---|---|---|---|---|---|
| MAPK | Genetic | Activating mutations in NRAS | Acquired | 18–23 | |
| Genetic | Activating mutations in KRAS | Acquired | 7 | ||
| Genetic | BRAF amplification | Acquired | 9–19 | ||
| Genetic | Activating mutations in MAP2K1 | Acquired | 4–7 | ||
| Genetic | Activating mutations in MAP2K2 | De novo and acquired | 9 | ||
| Genetic | Loss-of-function mutations in NF1 | De novo and acquired | 2 | ||
| Epigenetic | BRAF splicing | Acquired | 14–31 | ||
| PI3K/AKT | Genetic or epigenetic | Inactivating mutation, deletion, or loss of expression in PTEN | De novo and acquired | ∼9 | |
| Genetic | Activating mutations in PIK3CA or PIK3CG | Acquired | 4–6 | ||
| Genetic | Inactivating mutations in PIK3R2 | Acquired | 2 | ||
| Genetic | Activating mutations in AKT1 or AKT3 | Acquired | 4 | ||
| Others | Epigenetic | RTK overexpression or activation | Acquired | Not available | |
| Genetic | CDKN2A copy loss | De novo and acquired | 7 | ||
| Genetic | MITF amplification | Acquired | 2 |
Reactivation of the MAPK signaling pathway in resistance
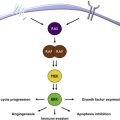
In normal cells, the MAPK pathway functions to transduce growth signal from receptors at the cell surface to transcriptional machinery in the nucleus, and it accomplishes this task through RAS guanosine triphosphatases (GTPases) and the serine/threonine kinases RAF, mitogen-activated protein kinase kinase (MEK), and extracellular signal receptor kinase (ERK) ( Fig. 1 ). The MAPK pathway is constitutively activated by BRAF V600 mutant proteins. In a clinical trial with vemurafenib, only patients whose tumors demonstrated more than 80% inhibition of the MAPK pathway achieved a clinical response. This finding suggested that marked inhibition of the MAPK pathway is critical to the clinical efficacy of the BRAFi. This hypothesis is supported by analyses showing that 70% to 90% of tumors with acquired resistance are characterized by reactivation of downstream effectors of this pathway. Of note, all melanomas with acquired resistance that have been analyzed have retained the same BRAF V600 mutation that was present before treatment. However, several other molecular changes that reactivate the pathway have been identified ( Fig. 2 ).
Somatic mutations in the NRAS gene that affect residues G12, G13, or Q61 lock the NRAS protein in the active, guanosine triphosphate (GTP)-bound state, resulting in constitutive activation of the MAPK pathway. Activating NRAS mutations are detected in approximately 20% of treatment-naïve melanomas. NRAS mutations are generally mutually exclusive with BRAF V600 mutations, and mutant NRAS appears to predominantly use CRAF to activate MEK and ERK. In contrast to treatment-naïve tumors, BRAF V600 tumors with acquired resistance to BRAFi have NRAS mutations in 18% to 23% of cases. In preclinical studies, treatment with MEK inhibitors was able to suppress the growth and survival of BRAFi-resistant cells expressing mutant NRAS , suggesting that reactivation of the MAPK pathway, not other pathways implicated in RAS signaling, was the key mediator of resistance. In addition to NRAS , mutations in KRAS , another gene encoding a member of the RAS family of proteins, have been detected in 7% of BRAFi-resistant tumors.
Although secondary mutations in BRAF have not been linked with BRAFi resistance, other alterations to the BRAF V600 allele have been implicated, such as amplification and aberrant splicing. The only study that has systematically examined both the DNA and the RNA from BRAFi-resistant melanomas found that amplification (∼18%) and erroneous splicing of BRAF V600 (∼14%) exist in a mutually exclusive fashion in 32% of tumors with acquired resistance, suggesting BRAF V600 alteration as the leading cause of BRAFi resistance. In addition, increased BRAF V600 expression attributable to epigenetic mechanisms has also been shown to mediate BRAFi resistance in patient-derived xenografts. BRAFi resistance mediated by amplification of the BRAF V600 allele can be countered by elevating the dose of BRAFi or combining the BRAFi with a MEK inhibitor (MEKi), and dosing BRAFi intermittently seems to prevent the emergence of resistant clones overexpressing BRAF V600 through epigenetic mechanisms. By contrast, alternative splicing results in the expression of truncated BRAF V600 proteins that lack the N-terminal RAS-binding domain but retain the kinase domain. The shortened BRAF V600 proteins form homodimers that are resistant to BRAFi, and the resistance cannot be overcome by increasing the concentration of the BRAFi. However, such cells remain dependent on activation of the MAPK pathway, as they are sensitive to MEK and/or ERK inhibition.
Possible gain-of-function mutations in genes encoding MEK1 and MEK2 ( MAP2K1 and MAP2K2 ) have been identified in 8% of BRAFi-naïve melanomas. Whereas some mutations in MEK1 and MEK2 have been identified in pretreatment tumor biopsies of patients and may not confer BRAFi resistance (eg, MEK1 I111S , MEK1 P124S , MEK1 P124L ), others have been detected only in samples with acquired resistance. Mutations associated with resistance include MEK1 K57N , MEK1 Q56P , MEK1 V60E , MEK1 C121S , MEK1 G128V , MEK1 E203K , MEK2 V35M , MEK2 L46F , MEK2 C125S , and MEK2 N126D . The incidence of activating mutations in MEK1 and MEK2 in tumors with acquired resistance is 7% to 16%. Activating mutations of MEK1/2 may also bestow resistance to allosteric MEK inhibitors, but cells expressing such mutations remain sensitive to ERK inhibition.
Neurofibromin 1 (NF1) negatively regulates RAS activity by promoting the hydrolysis of RAS-bound GTP to guanosine diphosphate. In addition to gene copy loss and loss-of-function mutations, NF1 function may be lost in melanoma because of excessive proteasomal degradation. Depletion of NF1 elevated HRAS, KRAS, and CRAF activities in BRAF V600E melanoma cells, which restored ERK reactivation in the presence of BRAFi. NF1-null cells were resistant to not only BRAFi monotherapy but also the combined inhibition of BRAF V600 and MEK1/2. However, NF1 loss did not alter the sensitivity to an ERK inhibitor. NF1 nonsense mutations (∼2%, NF1 R2450* ) and putative splice site mutations ( NF1 T135C , NF1 G4023A , and NF1 C3018T ) were identified in tumors from patients who had either a short PFS or experienced a relapse after initial response, suggesting that loss of NF1 function may be associated with both de novo and acquired BRAFi resistance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree