Gastrointestinal stromal tumors (GISTs) are unique tumors, arising largely due to oncogenic mutations in KIT or PDGFRA tyrosine kinases. Although surgery remains the most effective treatment, the remarkable clinical success achieved with kinase inhibition has made GIST one of the most successful examples of targeted therapy for the treatment of cancer. The insight gained from this approach has allowed a deeper understanding of the molecular biology driving kinase dependent cancers, and the adaptations to kinase inhibition, linking genotype to phenotype. Mutation tailored kinase inhibition with second generation TKI’s, and combination immunotherapy to harness the effects of TKIs remain exciting areas of investigation.
Key points
- •
Gastrointestinal stromal tumors (GISTs) are unique solid tumors because they are driven predominantly by oncogenic mutations in KIT or PDGFRA tyrosine kinases.
- •
Surgery is the most effective treatment for localized, primary GIST. Adjuvant tyrosine kinase inhibition (TKI) with imatinib substantially decreases recurrence rates but does not seem to affect overall survival.
- •
Imatinib is initial therapy for metastatic GIST; however, acquired mutations frequently lead to resistance after initial responses. The role of surgery and TKI in metastatic GIST remains unclear.
- •
Imatinib dose escalation, sunitinib, and regorafenib are the initial therapeutic options for imatinib-resistant GIST, with many novel TKIs under investigation.
- •
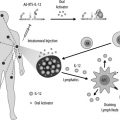
Preclinical data suggest that antitumor effects of imatinib in GIST are partially dependent on host immune responses. Combination imatinib and immunotherapy may be effective in GIST and other solid tumors.
Introduction
Gastrointestinal stromal tumor (GIST) is the most common sarcoma, accounting for approximately 18% of all sarcomas and 1% of all intestinal neoplasms. The annual incidence of GIST as determined by population-based studies is approximately 10 cases per million. GISTs have historically portended a poor prognosis. Up to 50% of patients have recurrent disease 5 years after complete resection. Median survival in metastatic GIST used to be approximately 9 months because it is inherently resistant to chemotherapy and radiation. The discovery of oncogenic tyrosine kinase mutations in GIST, and the successful application of kinase inhibitor therapies, have made GIST a model of targeting aberrant signal transduction to treat cancer. Lessons learned from this approach have allowed new insight into the molecular biology and mechanisms of resistance of kinase-driven cancers. It has spurred development of novel targeted inhibitors and uncovered exciting possibilities for combination therapy with other systemic agents.
Introduction
Gastrointestinal stromal tumor (GIST) is the most common sarcoma, accounting for approximately 18% of all sarcomas and 1% of all intestinal neoplasms. The annual incidence of GIST as determined by population-based studies is approximately 10 cases per million. GISTs have historically portended a poor prognosis. Up to 50% of patients have recurrent disease 5 years after complete resection. Median survival in metastatic GIST used to be approximately 9 months because it is inherently resistant to chemotherapy and radiation. The discovery of oncogenic tyrosine kinase mutations in GIST, and the successful application of kinase inhibitor therapies, have made GIST a model of targeting aberrant signal transduction to treat cancer. Lessons learned from this approach have allowed new insight into the molecular biology and mechanisms of resistance of kinase-driven cancers. It has spurred development of novel targeted inhibitors and uncovered exciting possibilities for combination therapy with other systemic agents.
Oncogenic kinase mutations and GIST pathogenesis
KIT
In 1998, 2 important discoveries were made that furthered our understanding of GIST biology. Hirota and colleagues described their landmark discovery of gain-of-function mutations in KIT in 5 patients with GIST. They hypothesized that these were oncogenic driver mutations, because Ba/F3 lymphoid cells transfected with mutant KIT cDNA underwent malignant transformation. Shortly thereafter, 2 groups reported that 95% of GISTs are immunohistochemically positive for the receptor tyrosine kinase KIT, also known as CD117. Since then, a causal relationship between KIT mutations and GIST pathogenesis has been further supported by many lines of evidence. Mutant KIT induces constitutive kinase activation without ligand binding. KIT mutations have been discovered in very small GISTs, suggesting that it occurs as a very early event. GIST tumor extracts almost universally show phosphorylated KIT. Transgenic Kit knock-in mouse models develop spindle cell tumors that are morphologically similar to human GIST. KIT blockade in vitro and in vivo inhibits tumor growth.
KIT, a receptor tyrosine kinase, binds KIT ligand (stem cell factor), which results in receptor dimerization, phosphorylation, and activation of downstream signaling pathways that promote cell proliferation and survival. It is now known that 70% to 80% of GISTs harbor a KIT mutation that induces constitutive kinase activation. Mutations most commonly occur in the juxtamembrane domain in exon 11 ( Fig. 1 , Table 1 ), which normally inhibits the kinase activation loop in the absence of ligand binding. Exon 11 mutations include in-frame deletions, insertions, and substitutions, but deletions are the most common. Mutations also occur in the extracellular domains (exons 8 [rarely] and 9), and infrequently in the kinase domains (exons 13 and 17) (see Fig. 1 , see Table 1 ). The downstream signaling pathways activated include the MAPK, PI3K-AKT, and STAT3 pathways, which lead to inhibition of apoptosis and cell proliferation. Recently, ETV1, a lineage survival factor in interstitial cells of Cajal, the hypothesized cell of origin for GIST, was shown to cooperate with activated KIT to induce GIST tumorigenesis.
| Gene | Incidence (%) | Anatomic Location | Imatinib Sensitivity |
|---|---|---|---|
| Mutations in KIT (80%) | |||
| Exon 9 | 7 | Small intestine, colon | Yes, consider 800 mg/d |
| Exon 11 | 65 | All locations | Yes |
| Exon 13 | 1 | All locations | Variable |
| Exon 17 | 1 | All locations | Variable |
| Mutations in PDGFRA (5%–8%) | |||
| Exon 12 | 2 | All locations | Yes |
| Exon 14 | <1 | Stomach | Yes |
| Exon 18 | 7 | Stomach, mesentery, omentum | D842V insensitive, most other sensitive |
| Wild Type (12%–15%) | |||
| BRAF V600E | 7–15 a | Stomach, small intestine | Possibly |
| SDHA, SDHB, SDHC, SDHD | 12 a | Stomach, small intestine | Usually not |
| Familiar GIST | |||
| KIT, rarely PDGFRA | Very rare | Small intestine | Usually not |
| Syndromic GIST | |||
| Unknown gene (Carney triad) | Very rare | Stomach | Usually not |
| SDHB, SDHC, SDHD (Carney-Stratakis) | Rare | Stomach | Usually not |
| NF1 (neurofibromatosis 1) | Rare | Small intestine | Usually not |
Platelet-Derived Growth Factor Receptor α
Approximately one-third of GISTs that do not have a mutation in KIT (8% of all GISTs) harbor a mutation in a closely related tyrosine kinase, platelet-derived growth factor receptor α (PDGFRA). PDGFRA and KIT mutations are mutually exclusive in GIST. Like mutations in KIT, PDGFRA mutations are found in its juxtamembrane domain (see Fig. 1 , see Table 1 ), adenosine triphosphate–binding domain, or activation loop, and cause ligand-independent receptor activation. An oncogenic role for these mutations in GIST has followed evidence similar to that for KIT: mutant PDGFRA induces ligand-independent receptor activation, and PDGFRA inhibition induces cellular arrest. However, PDGFRA mutant GISTs do have unique clinical profiles, including gastric location, epithelioid morphology, variable KIT expression, and a more indolent clinical course.
Wild-Type GIST
10% to 15% of tumors do not have mutations in KIT and PDGFRA (wild-type [WT] GIST). Other mutations that may contribute to tumorigenesis have been recently uncovered (see Table 1 ). Similar to BRAF mutations in melanoma, papillary thyroid cancer, and colorectal cancer, GIST BRAF mutations have also been identified in 7% to 15% of WT GISTS within the exon 15 V600E hot-spot. BRAF proteins and constituents of the MAPK signaling pathway can stimulate cell growth independent of KIT and are a possible cause of resistance to KIT and PDGFRA kinase inhibitors. Mutations in the succinate dehydrogenase (SDH) respiratory chain complex have also been discovered in WT GIST. SDH mutations were initially identified in the germline in subunits SDHB, SDHC, and SDHD, predisposing affected individuals to GIST and paraganglionomas (Carney-Stratakis syndrome). SDH mutations have since been identified in 12% of WT GIST (see Table 1 ). Mutations in SDHA have also since been reported. The precise oncogenic role of SDH mutations in GIST remains to be elucidated. Expression of insulin-like growth factor 1 receptor, which signals through MAPK and PI3K-AKT pathways, has also been detected and may contribute to GIST pathogenesis. WT GISTs are also found in 7% of patients with neurofibromatosis type I, who harbor germline mutations in the neurofibromin 1 gene (see Table 1 ).
Targeting kinase pathways in GIST
Until 2000, outcomes in patients with metastatic GIST were poor. Median survival was approximately 9 months, and responses to conventional chemotherapy was less than 5%. The discovery of oncogenic KIT mutations in GIST coincided with the successful clinical development and application of the tyrosine kinase inhibitor (TKI) imatinib (Gleevec) for the treatment of chronic myelogenous leukemia. It was noted that the kinases KIT and ABL shared structural similarity, prompting the first clinical application of imatinib in a 50-year-old woman with advanced GIST, which was met with a dramatic clinical response. This experience led to phase I, II, and 2 international phase III trials to investigate the benefit of imatinib in the metastatic setting. Overall, imatinib achieved disease control in 70% to 85% of patients with KIT-positive GIST, with a median progression-free-survival of 20 to 24 months, and an estimated overall survival (OS) greater than 36 months ( Fig. 2 ). The advent of imatinib therapy for metastatic GIST has dramatically altered prognosis: median survival is 5 years with 34% of patients surviving more than 9 years. Imatinib is first-line treatment in patients with metastatic GIST, and treatment is recommended to continue indefinitely if there is clinical benefit, because interruption is associated with high rate of relapse.
Paralleling the success in GIST, a molecular approach to systemic therapy has been adopted in many other solid tumors. Genomic analyses have uncovered biologically relevant and druggable kinase mutations in other solid malignancies. Although the success achieved in these cancers has not replicated the GIST success, it has validated a molecular approach to systemic treatment and has heralded kinase-based therapies as an integral component of cancer care ( Table 2 ).
| Gene | Tumor | Agent |
|---|---|---|
| KIT | Melanoma, seminoma, small cell lung cancer, synovial sarcoma, thymic carcinoma | Imatinib |
| PDGFRA | Dermatofibrosarcoma protuberans | Imatinib |
| EGFR | Non–small cell lung cancer Squamous cell, ovarian, renal cell, and colorectal cancer, glioblastoma multiforme | Gefitinib, erlotinib Erlotinib, gefitinib, lapatanib, cetuximab Panitumumab |
| BRAF | Melanoma, papillary thyroid cancer, colon cancer | Vemurafenib |
| HER-2 | Breast cancer, lung cancer | Trastuzumab |
| VEGFR | Non–small cell lung, breast, prostate, renal, colorectal | Bevacizumab, vascular endothelial growth factor inhibitors |
| RET | Multiple endocrine neoplasia 2A, 2B, familial medullary thyroid cancer, radiation-associated papillary thyroid cancer | Cabozantinib, vandetanib, sorafenib |
Assessing response to kinase therapy
Responses to systemic therapy in solid tumors have traditionally been assessed using the response evaluation criteria in solid tumors (RECIST), which incorporates unidirectional tumor size. However, assessing responses using RECIST has been shown to be insensitive in GIST. Positron emission tomography (PET) scans had traditionally been used to assess continuing responses to TKI treatment, because significant decreases in fluorodeoxyglucose signal are seen within 24 hours in patients responding to imatinib. However, Choi and colleagues proposed using computed tomography to determine tumor size and density in assessing treatment response; responding tumors show homogeneous and hypodense features, losing solid elements and neovascularity. The Choi criteria correlate with PET, are superior to RECIST, and are a significant improvement in our understanding of assessing clinical responses to systemic agents in solid tumors.
Combining targeted therapy with surgery
Adjuvant Imatinib
Although TKI therapy induces tumor regression in most patients, it rarely induces complete responses. Even long-term TKI therapy fails to eradicate GIST cells, with viable tumor cells detected even in tumors with good histologic responses. In contrast, surgery for patients with primary GIST without metastases cures more than 50% of patients. In a double-blind, placebo-controlled, multicenter, randomized trial, the American College of Surgeons Oncology Group (ACOSOG) reported that 1 year of adjuvant imatinib after resection of GISTs at least 3 cm in size significantly improved 1-year recurrence-free survival (RFS) (83% in placebo arm vs 98% in imatinib arm, Fig. 3 ). Based on these results, the US Food and Drug Administration (FDA) approved imatinib for use in the adjuvant setting. Recently, it was shown that patients at high risk of recurrence treated with 3 years of adjuvant imatinib after surgical resection have 5-year RFS and OS rates of 65.6% and 92%, respectively, compared with 47.9% and 81.7% in patients treated with 1 year of adjuvant imatinib. However, there was no difference in disease-specific survival between 1 and 3 years of therapy. An additional phase III trial is examining the outcomes after 2 years of adjuvant imatinib after surgery. A phase II, nonrandomized, multicenter trial is also evaluating the efficacy of 5 years of adjuvant imatinib after complete resection of primary GIST. The success of adjuvant imatinib in GIST ranks with trastuzumab as one of the most successful applications of kinase inhibitor therapy for the adjuvant treatment of solid tumors.
Neoadjuvant Imatinib
When primary GIST seems borderline resectable or unresectable, neoadjuvant imatinib treatment may allow for tumor shrinkage and a subsequent R0 resection. Preliminary phase II trials have shown the safety and efficacy of preoperative imatinib. However, there are no published phase III data on neoadjuvant imatinib for unresectable GIST. This is an area of ongoing investigation.
Molecular biology and risk stratification
Similar to other sarcomas, tumor size, mitotic index, and location have been shown to determine biological aggressiveness in GIST. However, the discovery of oncogenic kinase mutations has allowed new insight into links between molecular biology and clinical behavior. It is now clear that recurrence patterns after primary resection are also governed by mutation type: deletion and insertion mutations in KIT exon 11 and exon 9 confer higher recurrence rates compared with other mutations. Within exon 11 mutations, deletions (specifically in amino acids 557 or 558) have worse outcome. Our understanding of risk stratification to predict the natural history of resected disease is achieved through prognostic nomograms. We developed a nomogram predicting 2-year and 5-year RFS factoring tumor size, mitotic index, and location ( Fig. 4 ). Dei Tos and colleagues have reported a nomogram predicting 10-year OS. The relationship between mutation type, adjuvant imatinib, and other factors in the nomogram remain unclear.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree