Fig. 11.1
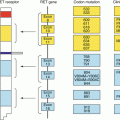
MAPK pathway, TKI receptor inhibition MAPK mitogen-activated protein kinase TK tyrosine kinase
Fifty per cent of cases of sporadic MTC result from somatic mutations in the RET gene. The hereditary forms of MTC, including the multiple endocrine neoplasia (MEN) 2A, MEN2B syndromes and familial MTC result from germline mutations in different codons of the RET gene. In MTC, a substitution of a single nucleotide can activate the RET gene, resulting in unencumbered activation of the TK receptor. In familial MTC and MEN2A, the codons commonly mutated are 609, 611, 618 and 620, whereas in MEN2B codon 918 is most common and elicits an especially aggressive clinical pattern. In sporadic MTC, codon 918 is also the most often mutated [3].
Oncogenic activation of BRAF results in more aggressive DTC. The RAF kinase in mammalian cells has three different isoforms—A, B and C. The BRAF kinase isoform is expressed in haematopoietic cells, neurons, testis cells and thyroid follicular cells [14, 15]. The V600E transversion is the most common mutation in BRAF, in which a thymine to adenine transversion occurs at position 1799, resulting in a valine to glutamate substitution at position 600 [14]. The V600E mutation renders BRAF constitutively active. BRAF mutation is the most common genetic change in PTC. Although RET/PTC and RAS mutations are also common, they are independent of each other. Patients with BRAF mutations with PTC have more aggressive properties than PTC without mutations, including a greater likelihood of extrathyroidal invasion and tall-cell variant [16]. Furthermore, normal BRAF produces proteins that aid in follicular cell functions, including the sodium-iodide symporter and the TSH receptor [17]. Thus, when BRAF is mutated, these differentiated functions are diminished. In clinical practice, patients with BRAF mutations have more radio-resistant disease, present with cancers at a more advanced stage, and have a higher incidence of recurrence and mortality [17].
Also targeted in DTC are processes that stimulate tumour growth and development, such as angiogenesis. The VEGFR is activated by other cytokines, hormones and growth factors that stimulate components of MAPK signalling and promote angiogenesis (Fig. 11.2) [17]. Several subtypes of the VEGFR can induce tumour growth and proliferation. With regard to DTC and MTC, the greater the VEGFR expression, the higher the risk of metastasis and recurrence [18].
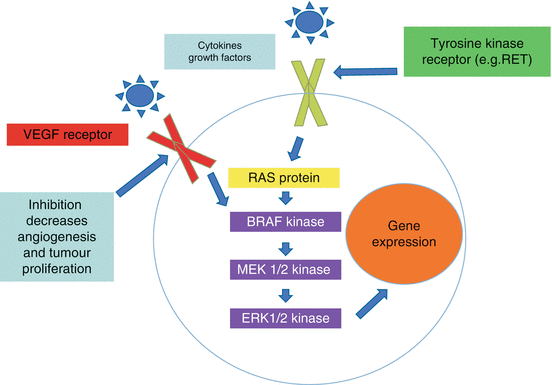

Fig. 11.2
MAPK pathway, VEGF receptor inhibition MAPK mitogen-activated protein kinase VEGF vascular endothelial growth factor
Inhibitors of VEGFR could act by limiting angiogenesis and the growth of thyroid cancer by restricting the blood supply [18]. These VEGFR inhibitors are thought to stabilize tumour progression more so than preventing tumour initiation.
Patient Selection for Targeted Therapy
Determining the proper candidates is critical to optimizing the clinical utility of these novel therapies. In DTC, patients with non-RAI-avid thyroid cancer who are potential candidates for targeted therapy must be comprehensively evaluated on the basis of key clinical factors, including their age and performance status, the extent of disease and the rate of disease progression [19]. Indeed, targeted therapy relies upon patient selection, target identification and specific inhibition of oncogenic cell pathways. In DTC, to be considered for targeted therapy, a patient must have RAI-refractory disease, defined as having at least one lesion without RAI uptake, or those that have progressed within a year after RAI treatment [19]. The overall goal of targeted cancer therapy is to treat tumour cells by using drugs that target the molecular pathways that give rise to tumours, but leave normal cells unaffected. DTC patients have thyroglobulin-positive disease that is non-RAI-avid—meaning the DTC was detected using other imaging modalities. In patients with metastatic thyroid cancer that is relatively stable, based on tumour markers and radiological evaluation, the administration of targeted therapy is questionable, given the relative stability and lower benefit compared with those patients with more aggressive tumour biology. Good candidates for targeted therapy include people pf older age with a poorly differentiated thyroid tumour, with no or low RAI uptake (in DTC), large metastases located in the bones and not lungs, with avid uptake on fludeoxyglucose positron emission tomography (FDG-PET) scan, and rapidly progressive disease [19].
In MTC, patients with distant disease that is progressive and clinically symptomatic should be considered for systemic options, as RAI is not used. Calcitonin and carcinoembryonic antigen (CEA) levels as tumour markers are used to follow these patients, and their doubling times are calculated to determine the rate of progression of their disease. A doubling time of 6 months is thought to be rapid enough to warrant consideration of systemic therapy. Moreover, tumour location in the neck helps guide therapy. For instance, a patient with lateral neck disease that is asymptomatic with progressive distant disease is a reasonable candidate for systemic therapy. In cases of distant metastatic MTC with recurrent or persistent cancer in the central neck, there is concern for initiating systemic therapy, as the risks of doing so include bleeding (near the carotid arteries and jugular veins) and fistula formation (near the trachea or oesophagus). Moreover, patients who are placed on systemic therapies have a higher bleeding risk and consideration of any subsequent operative resection for neck disease will be delayed [20].
Patients with extensive metastatic disease that would potentially cause symptoms are also good candidates for targeted therapy. The speed of progression is evaluated and documented according to the RECIST criteria [21]. Patients with measurable tumours are radiologically monitored using these criteria with radiological testing. Although only a few such patients with thyroid cancer will be candidates for targeted therapy, the clear identification of these patients with reproducible criteria on serial imaging tests is paramount in their selection.
Biologically Targeted Therapies
An understanding of the molecular pathways leading to and promoting thyroid oncogenesis has led to novel systemic therapies, including but not limited to TK inhibitors (TKIs), RET, BRAF and VEGFR inhibitors. Clinical trials should be the first-line therapy for these patients who have systemic, progressive, non-RAI-avid tumours. If a trial is not available or if the patient is not suitable for a trial, off-label use of commercially available targeted therapies should be considered [22]. Available therapies include inhibitors of oncogenic signalling pathways, cell signalling and angiogenesis. TKIs inhibit transmembrane receptors that initiate signalling in the MAPK pathway (Fig. 11.1). TKIs are orally administered and are generally tolerated well [23]. Commercially available TKIs include sorafenib, sunitinib and pazopanib.
Drugs that are not commercially available but are used in clinical trials include motesanib and axitinib. Vemurafenib is a BRAF inhibitor; vandetanib is a RET kinase inhibitor used particularly in MTC [7, 17, 22]. Many of these therapies have inhibitory effects on multiple kinases, including VEGFR inhibition which stabilizes tumour progression (Fig. 11.2). Table 11.1 lists the drugs discussed in this chapter and their molecular targets. Individual side-effects of each drug are discussed, but one that is consistent in many of the therapies is the need to often readjust and increase thyroid hormone replacement in patients because of malabsorption and/or increased clearance while on targeted therapies. This is especially relevant in those patients with metastatic DTC, as TSH suppression is still an integral part of their treatment.
Table 11.1
Targeted therapies for thyroid cancer and their molecular targets
Drug | RET | BRAF | VEGFR | Other |
|---|---|---|---|---|
Sorafenib | X | X | 2, 3 | c-KIT |
Sunitinib | X | 1, 2, 3 | ||
Motesanib | X | 1, 2, 3 | c-KIT, PDGFR | |
Axitinib | 1, 2, 3 | c-KIT | ||
Pazopanib | 1, 2, 3 | c-KIT, PDGFR | ||
Vemurafenib | X | |||
Vandetanib | X | 2, 3 | RET/PTC3, sporadic/MEN2B RET mutations |
Sorafenib is a multi-kinase inhibitor of RET, BRAF, and VEGFRs 2 and 3. This drug is approved by the US Food and Drug Administration (FDA) for use only in advanced renal cell carcinoma (RCC) and unresectable hepatocellular carcinoma. Three phase II trials have shown benefit in DTC, with rates of PR of 15–25 % and stable disease (SD) rates at 1 year of 34–61 % [7, 24–27]. A single-institution study showed a clinical benefit of 80 % (20 % PR and 60 % SD) [23]. It further recognized a more pronounced response in lung metastases (22 %) than in nodal disease (0 %) [23]. The median progression-free survival (PFS) was 19 months [23]. As sorafenib has shown benefit in clinical trials, some clinicians have been using this drug as an off-label, non-FDA approved therapy in metastatic non-radioactive-avid thyroid cancer. For MTC, a phase II clinical trial evaluating sorafenib in metastatic MTC showed PR in 1 of 16 patients in the sporadic MTC group, and 14 of the 16 had SD [28]. One patient had PR for 21+ months while 4 patients had SD for ≥15 months. Median PFS was 17.9 months. The initial grouping for the study called for a hereditary arm and a sporadic arm; the hereditary arm was terminated early because of lack of accrual [28]. The side-effects of sorafenib include hand–foot syndrome, rash, fatigue, diarrhoea, QT prolongation, bleeding risks and hypertension. Dermatological changes, including keratoacanthomas and squamous cell carcinoma (SCC) are seen in ≤10 % of patients [25, 29].
Sunitinib is another oral, small molecule TKI that targets VEGFRs 1, 2, 3, and RET. It is FDA-approved for RCC and has been used off-label for metastatic DTC. This drug has shown a PR of 13 % in a phase II trial, with disease stabilization occurring in 68 % of patients, resulting in 81 % clinical benefit [7]. Another phase II study included patients with MTC and DTC and showed a RECIST response on FDG-PET scan in 8 of 29 patients with DTC and 3 of 6 patients with MTC (28 % and 50 %, respectively) [30]. Although the number of patients for MTC was small, a demonstrable decrease of 50 % was also seen in serum calcitonin levels [30]. Side-effects of this drug include fatigue, diarrhoea, palmar or plantar erythrodysesthesia, neutropenia and hypertension [7].
Motesanib is an oral TKI that targets VEGFRs 1, 2, 3, platelet-derived growth factor receptor (PDGFR), c-Kit, and RET. In one phase II study, patients with locally advanced, metastatic or non-radio-avid DTC showed an objective response of 14 % and SD in 67 % [31]. The median PFS was 40 weeks and 81 % patients showed decreased thyroglobulin levels compared to baseline [31]. Another phase II trial showed 81 % SD in a group of patients with progressive, advanced or metastatic MTC [32]. The objective response was low at 2 %. Median PFS was 48 weeks and among tumour marker analysis, an 83 % decrease occurred in serum calcitonin levels. The most common side-effects included diarrhoea, hypertensions and weight loss. Moreover, this drug was especially noted to require an increase in mean dosage of thyroid replacement [32]. Although objective response was low, disease stability was considerable in both studies.
Axitinib is a TKI that selectively inhibits VEGFRs 1, 2, and 3. One phase II trial looking at all histological subtypes (DTC, MTC and ATC) showed PR in 30 % of patients, SD in 38 % of patients, disease progression in 7 % of patients, while 25 % had an indeterminate response or did not undergo post-baseline scans [33]. On tumour assessment using RECIST criteria, of 45 patients with papillary of follicular histology, 14 had PR with a median follow-up of 16.6 months [33]. Nineteen of these 45 patients had SD, 3 had progressive disease, and 9 were deemed indeterminate since they did not meet any response criteria or did not have post-baseline scans [33]. For MTC, 2 of 11 patients had PR and 3 of 11 had SD [33]. Common side-effects of this drug include hypertension, stomatitis, fatigue and diarrhoea [22]. It is important to note that the efficacy of axitinib to induce objective responses and disease stability was in the absence of any anti-RET activity; this suggests that RET may not be as important a target for therapy as VEGFR [7].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree