Fig. 3.1
CTV delineation for a muscle-invasive bladder cancer patient treated with definitive IGRT
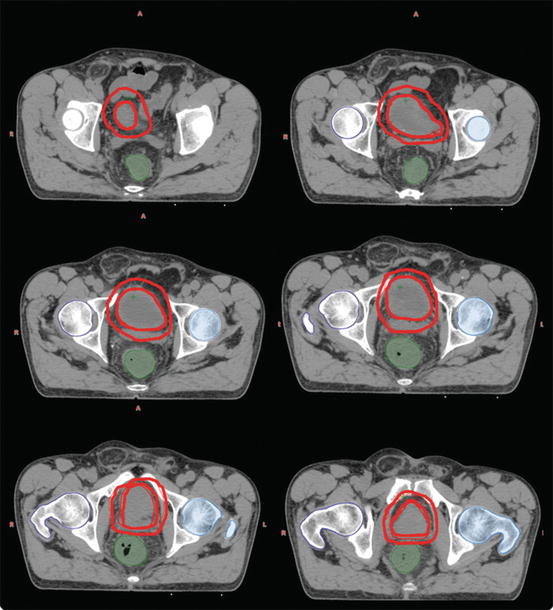
Fig. 3.2
Boost volume delineation for a muscle-invasive bladder cancer patient treated with definitive IGRT
3.4.3 Role of Elective Nodal Irradiation
There are conflicting data on elective nodal irradiation in the treatment of bladder cancer. The rate of LN involvement is approximately 25% according to radical cystectomy series, and this finding helps to indicate the need for extensive LN dissection (LND) and adjuvant chemotherapy [10, 21, 22]. The number of dissected LNs is directly correlated with the outcome of patients, independent of the N stage [23, 24]. Based on these findings, elective nodal irradiation may provide a survival benefit in patients that did not undergo LND whether they are involved or not, with the expense of additional toxicity. However, pelvic control and survival rates are not decreased with irradiation of the bladder alone when used together with chemotherapy [25].
The BC20001 study reported a low rate of nodal relapse both in patients that received RT alone (7%) and in patients that received chemoradiotherapy (5%) [26]. In a randomized trial which compares 45 Gy of whole pelvic RT including elective nodal irradiation and 20 Gy boost dose with bladder-only irradiation of 65 Gy, no difference was found in the rates of pelvic nodal relapse and overall survival in patients with complete response [27]. Therefore, it may be reasonable to electively irradiate the pelvic lymphatic regions in patients that cannot receive concurrent chemotherapy.
3.4.4 Planning Target Volume
Some authors define the whole bladder as PTV with a 1.5-cm margin to the uninvolved outer bladder wall and the extravesical extent of the tumor with a 2-cm margin. In other instances, the PTV is formed with a 2-cm margin to the CTV in order to adequately cover all errors based on setup and organ motion. It was shown that the bladder wall can move beyond 1.5 cm at least once during a course of treatment in over 60% of patients and the GTV can move outside the PTV on at least one course of treatment in over 20% of patients [28–30]. Graham et al. [31] recommend adding margins of 1.6 cm anteriorly and posteriorly, 1.4 cm laterally, 3 cm superiorly, and 1.4 cm inferiorly; however, this will certainly cause increased rate of radiation-related toxicity. Meijer et al. [32], on the other hand, recommend margins of 1 cm anteriorly and laterally, 1.2 cm inferiorly, 1.4 cm posteriorly, and 2 cm superiorly. To overcome this issue, daily image-guided therapy can be administered, or insertion of fiducial markers in the bladder wall around the tumor bed can be another solution. The use of these techniques can decrease the margins <1.5 cm [33]. However, besides the movement of the bladder, the change of its shape is also challenging. Therefore, margins for the PTV do not seem to be reduced at any time soon. The recommended margins for the PTV are 1–1.5 cm in all directions and 2–2.5 cm in the superior direction when using conventional RT [15]. The inferior margin can be reduced to 1 cm if the prostate or urethra has been included. One should also consider which walls of the bladder are involved by the cancer and the relative mobility of this particular portion of the bladder.
3.5 Adjuvant Setting
If surgery is planned to a patient with bladder cancer, RT may have a role in the pre- or postoperative setting. Preoperative RT has been questioned because of the fact that it delays the time to definitive surgery and increases its morbidity, as well as it may compromise the surgeon’s ability to perform continent urinary diversions. On the other hand, the survival benefit of preoperative RT was only shown for clinical T3 and T4 tumors [34, 35]. The only prospective randomized trial of postoperative RT which includes patients with transitional cell and squamous cell bladder cancer revealed increased local control and disease-free survival rates compared to observation after surgery [36]. However, the morbidity of postoperative RT is higher than preoperative RT because of the large volumes occupied by the small bowel after cystectomy. It was reported that the incidence of small bowel obstruction was >30% with 50 Gy in conventional fractionation [37]. Therefore, preoperative RT is a safer option; however, if postoperative RT is to be administered, dose reduction is recommended with a small RT field in order to minimize radiation-induced toxicity.
Conclusion
Patient-based treatment planning is the standard approach in bladder cancer RT. Recommended simulation and delineation techniques are summarized in this chapter. If these recommendations are applied in clinical practice, the variations in treatment techniques between institutions can be minimized.
References
1.
2.
3.
4.
5.
Coppin CM, Gospodarowicz MK, James K, Tannock IF, Zee B, Carson J, et al. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1996;14(11):2901–7.CrossrefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree