Estrogen Receptor–positive/HER-2 negative breast cancers represent a heterogeneous group of tumors. Luminal A and B tumor subtypes can be identified through immunohistochemical assessment of estrogen and progesterone receptor, Ki-67 and HER-2 status. Patients with high levels of expression of steroid hormone receptors and low proliferation (Luminal A) are commonly cured with endocrine therapy alone. Patients with doubtful endocrine responsiveness or with high proliferation index (Luminal B/Her-negative) require the addition of chemotherapy to the best endocrine therapy. Controversies still exist on the identification of those patients who do not benefit from chemotherapy. Tailored adjuvant treatments should be considered in the therapeutic algorithm of patients with luminal tumors.
Key points
- •
Luminal breast cancers are the most frequent subtypes; they are heterogeneous, with a large variation in clinical presentation.
- •
Luminal A tumors are characterized by estrogen-regulated genes and better outcomes, whereas luminal B tumors are characterized by a higher genomic grade and poorer outcomes.
- •
Subsets of patients with high levels of expression of estrogen receptor (ER) with low proliferation (luminal A) are commonly cured with endocrine therapy alone; subsets with doubtful endocrine responsiveness and/or with high proliferation index (luminal B) require the addition of chemotherapy.
- •
Recent recommendations suggest the use of standard pathology reports based on the immunohistochemical (IHC) measurements of ER, progesterone receptor (PgR), Ki67, and HER2 as a surrogate molecular classification to identify subgroups of patients who might avoid/receive chemotherapy.
- •
Great advances have been made in the development of multigene prognostic signatures for gene expression patterns to identify subset of patients with luminal breast cancers who benefit from adding chemotherapy to endocrine therapy.
- •
Much attention should be paid to the identification of biomarkers in addition to hormone receptor expression and proliferation indexes to better select those patients who would benefit from endocrine therapy.
Introduction
Luminal breast cancers are traditionally the most frequent subtypes of breast cancer, representing a heterogeneous group of tumors with a large variation in clinical presentation. Selection of patients who might forego chemotherapy within these subgroups represents a major question.
Controversies concern groups with bulky tumors and/or positive nodes who present with favorable biology (ideally luminal A) versus patients with small and node-negative tumors but with an unfavorable biology (ideally biologically aggressive luminal B). Among smaller tumors traditionally judged as low risk and not requiring chemotherapy, there is a not negligible subgroup with adverse tumor biology that should be considered for a combined chemoendocrine therapeutic approach. Within this subgroup, the cross talk with other pathways should be also considered and new strategies to escape multipathway drivers of tumor growth and progression should be implemented. Novel agents to be added to endocrine therapy are under investigation to shut down bypass signaling pathways that drive tumor growth.
Similarly, among subsets defined by high levels of expression of ER along with low proliferation, commonly cured with endocrine therapy alone, there is a subgroup presenting with very large tumor burden and a related dismal prognosis that might require the addition of chemotherapy.
Care for patients through a personalized approach remains a central issue, and clinicians are currently more likely to give selective interventions to minimize acute and late toxicity without compromising efficacy. In particular, for the subgroup of patients with ER-positive disease, appropriate adjuvant systemic therapy involves choosing treatments tailored to individual patients according to biologic features and assessments of patient risk but also according to comorbidities, desire to preserve fertility, and patient preference.
Recommendations from the St. Gallen consensus panel suggested the use of standard pathology reports based on IHC measurements of ER, PgR, Ki67, and HER2 as a surrogate molecular classification to identify subgroup of patients who might avoid/receive chemotherapy. Common features that might indicate increased response to endocrine therapy include high expression of ER and PgR. Conversely, the presence of high proliferation index and/or lower expression of steroid hormone receptors might identify a subgroup of patients with luminal disease at higher probability of response to chemotherapy.
This clinicopathologic classification requires the availability of reliable measurements of its individual components, therefore guidelines have been published for ER and PgR determination and for the proper evaluation of Ki67.
In parallel with clinicopathologic classification, a great advance was reached in the development of multigene prognostic signatures for gene expression patterns to identify a subset of patients with luminal breast cancers who benefit from adding chemotherapy to endocrine therapy.
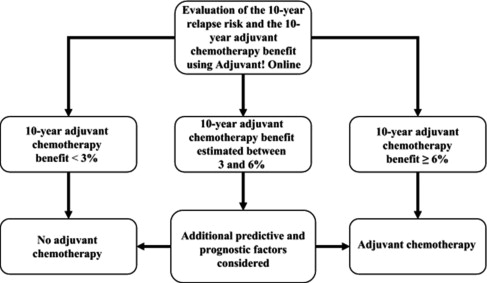
In particular, the fine tuning for prediction of which groups may benefit from endocrine therapy alone has been possible with the advent of multigene assays, such as the 21-gene recurrence score (RS) and 70-gene assays, because the findings from the retrospective analysis of 2 large trials (SWOG S8814 and NSABP [National Surgical Adjuvant Breast and Bowel Project] B-20) have shown that patients with low RS may be sufficiently treated with endocrine therapy alone. A popular method for assessing the magnitude of benefit of endocrine therapy and chemotherapy in luminal breast cancer is Adjuvant! Online ( Fig. 1 ). The algorithm used, however, is based on historical trials with biologic features evaluated in an older fashion, in which the comparator groups may not have received adequate endocrine therapy.
Prospective validation of these multigene signatures is under way. Specifically, the Trial Assigning Individualized Options for treatment (Rx) (TAILORx) (ClinicalTrials.gov identifier NCT00310180 ) is studying the utility of the Oncotype DX score to predict chemotherapy benefit in the intermediate score subgroup.
The Microarray in Node-negative and 1 to 3 Positive Lymph Node Disease May Avoid Chemotherapy Trial (MINDACT) (ClinicalTrials.gov identifier NCT00433589 ) is studying the outcomes of patients with discordant risk assessments using the 70-gene expression signature of MammaPrint compared with clinicopathologic features with the Adjuvant! Online program. The RxPONDER ([Rx for Positive Node, Endocrine Responsive Breast Cancer] ClinicalTrials.gov identifier NCT01272037 ) is a large prospective trial designed to determine the benefit of chemotherapy among women with hormone receptor–positive breast cancer, 1 to 3 positive nodes, and an RS less than or equal to 25, as determined by Oncotype DX testing. The results from these large, prospective studies are eagerly awaited and will hopefully shed light on the best strategies to personalize adjuvant treatment in patients with luminal breast cancer.
This article discusses the evolving knowledge of adjuvant treatments in order to define reasonable but also novel treatment strategies in patients with ER-positive/HER2-negative (luminal A and luminal B) breast cancer.
Introduction
Luminal breast cancers are traditionally the most frequent subtypes of breast cancer, representing a heterogeneous group of tumors with a large variation in clinical presentation. Selection of patients who might forego chemotherapy within these subgroups represents a major question.
Controversies concern groups with bulky tumors and/or positive nodes who present with favorable biology (ideally luminal A) versus patients with small and node-negative tumors but with an unfavorable biology (ideally biologically aggressive luminal B). Among smaller tumors traditionally judged as low risk and not requiring chemotherapy, there is a not negligible subgroup with adverse tumor biology that should be considered for a combined chemoendocrine therapeutic approach. Within this subgroup, the cross talk with other pathways should be also considered and new strategies to escape multipathway drivers of tumor growth and progression should be implemented. Novel agents to be added to endocrine therapy are under investigation to shut down bypass signaling pathways that drive tumor growth.
Similarly, among subsets defined by high levels of expression of ER along with low proliferation, commonly cured with endocrine therapy alone, there is a subgroup presenting with very large tumor burden and a related dismal prognosis that might require the addition of chemotherapy.
Care for patients through a personalized approach remains a central issue, and clinicians are currently more likely to give selective interventions to minimize acute and late toxicity without compromising efficacy. In particular, for the subgroup of patients with ER-positive disease, appropriate adjuvant systemic therapy involves choosing treatments tailored to individual patients according to biologic features and assessments of patient risk but also according to comorbidities, desire to preserve fertility, and patient preference.
Recommendations from the St. Gallen consensus panel suggested the use of standard pathology reports based on IHC measurements of ER, PgR, Ki67, and HER2 as a surrogate molecular classification to identify subgroup of patients who might avoid/receive chemotherapy. Common features that might indicate increased response to endocrine therapy include high expression of ER and PgR. Conversely, the presence of high proliferation index and/or lower expression of steroid hormone receptors might identify a subgroup of patients with luminal disease at higher probability of response to chemotherapy.
This clinicopathologic classification requires the availability of reliable measurements of its individual components, therefore guidelines have been published for ER and PgR determination and for the proper evaluation of Ki67.
In parallel with clinicopathologic classification, a great advance was reached in the development of multigene prognostic signatures for gene expression patterns to identify a subset of patients with luminal breast cancers who benefit from adding chemotherapy to endocrine therapy.
In particular, the fine tuning for prediction of which groups may benefit from endocrine therapy alone has been possible with the advent of multigene assays, such as the 21-gene recurrence score (RS) and 70-gene assays, because the findings from the retrospective analysis of 2 large trials (SWOG S8814 and NSABP [National Surgical Adjuvant Breast and Bowel Project] B-20) have shown that patients with low RS may be sufficiently treated with endocrine therapy alone. A popular method for assessing the magnitude of benefit of endocrine therapy and chemotherapy in luminal breast cancer is Adjuvant! Online ( Fig. 1 ). The algorithm used, however, is based on historical trials with biologic features evaluated in an older fashion, in which the comparator groups may not have received adequate endocrine therapy.
Prospective validation of these multigene signatures is under way. Specifically, the Trial Assigning Individualized Options for treatment (Rx) (TAILORx) (ClinicalTrials.gov identifier NCT00310180 ) is studying the utility of the Oncotype DX score to predict chemotherapy benefit in the intermediate score subgroup.
The Microarray in Node-negative and 1 to 3 Positive Lymph Node Disease May Avoid Chemotherapy Trial (MINDACT) (ClinicalTrials.gov identifier NCT00433589 ) is studying the outcomes of patients with discordant risk assessments using the 70-gene expression signature of MammaPrint compared with clinicopathologic features with the Adjuvant! Online program. The RxPONDER ([Rx for Positive Node, Endocrine Responsive Breast Cancer] ClinicalTrials.gov identifier NCT01272037 ) is a large prospective trial designed to determine the benefit of chemotherapy among women with hormone receptor–positive breast cancer, 1 to 3 positive nodes, and an RS less than or equal to 25, as determined by Oncotype DX testing. The results from these large, prospective studies are eagerly awaited and will hopefully shed light on the best strategies to personalize adjuvant treatment in patients with luminal breast cancer.
This article discusses the evolving knowledge of adjuvant treatments in order to define reasonable but also novel treatment strategies in patients with ER-positive/HER2-negative (luminal A and luminal B) breast cancer.
Specific consideration for adjuvant treatment choice in luminal A tumors
Based on retrospective analysis of past trials, prospectively stratified for the degree of ER expression, the effect of chemoendocrine therapy on luminal A patients compared with endocrine therapy alone is unclear. The evidence in node-negative disease comes from the International Breast Cancer Study Group (IBCSG) trial IX for postmenopausal women and from IBCSG trial VIII for premenopausal patients.
The trials compared 3 or 6 courses of adjuvant classical cyclophosphamide, methotrexate, and fluorouracil (CMF) with or without endocrine therapy versus endocrine therapy alone. No clear chemotherapy benefit was observed in ER-positive/HER2-negative disease (hazard ratio [HR] 0.90; 95% CI, 0.74–1.11). Moreover, these 2 trials showed no benefit of chemotherapy in the subset of ER positive, HER2 negative, and low Ki67, corresponding to the surrogate definition of luminal A disease.
In a retrospective analysis of the NSABP B-20 trial, the Oncotype Dx RS was used to predict CMF chemotherapy benefit. It was demonstrated that patients with ER-positive/node-negative breast cancer treated with tamoxifen and with a low RS did not benefit from the addition of chemotherapy. Analogous findings were reported by Albain and colleagues, in a retrospective analysis of the SWOG 8814 trial, where patients with ER-positive/node-positive disease had no benefit from adding cyclophosphamide, doxorubicin, and fluorouracil regimen to tamoxifen if low to intermediate RS was demonstrated. Clinicians in favor of the inclusion of chemotherapy might argue, however, against these conclusions, considering the retrospective nature of the studies, the small number of cases that were available for inclusion, and the lack of a taxane in the delivered chemotherapy.
According to the recent St Gallen consensus conferences, the intrinsic luminal A subtype corresponds to the clinicopathologic surrogate definition as luminal A–like. These luminal A tumors are characterized by high expression of steroid hormone receptors and are HER2 negative and Ki67 low. The optimal cutpoint between high and low values for Ki67 has not been established, given the potential variability of assessment among different laboratories.
A level of Ki67 less than 14% best correlated with gene array definition of luminal A, based on results in a single reference laboratory. Recent data support a threshold of 20% or higher as potentially indicative of high Ki67 status.
Literature data recently also sustain the introduction of PgR to optimally distinguish between luminal A and luminal B tumors. The value of PgR suggested for categorizing luminal A and luminal B subtypes is proposed as more than 20%, according to a recent report, where the fine tuning was done by comparing the multigene expression–based assays across 5 independent cohorts and the IHC-based definition of luminal A and luminal B.
In conclusion, the threshold for the definition of luminal disease remains a matter of debate.
Tailored Treatments of Luminal A Disease
The role of endocrine therapy in luminal A breast cancer is well consolidated.
Tamoxifen for 5 years can be considered the default adjuvant endocrine therapy for premenopausal patients.
Recently, there is evidence that extension of endocrine treatment beyond 5 years or even 10 years might be beneficial (as reported by the Adjuvant Tamoxifen—Longer Against Shorter [ATLAS] study, which suggested a significant benefit for extending tamoxifen to 10 years rather than 5 years), although not required, in a large group of luminal A patients. Adding ovarian suppression to tamoxifen is a matter of controversy, especially for younger patients (<40 years), and results from the recently concluded Suppression of Ovarian Function with Triptorelin (SOFT) trial will add some information to this issue. Ovarian suppression alone without tamoxifen, as well as its combination with aromatase inhibitors, should not be routinely considered unless tamoxifen is contraindicated.
Aromatase inhibitors for 5 years should be considered the mainstay of treatment for the vast majority of postmenopausal women with luminal A disease, although this strategy should be preferred for patients at high risk, such as those with luminal B disease.
There are some postmenopausal women with luminal A disease who could be treated with tamoxifen alone. Extension of aromatase inhibitor therapy beyond the first 5 years for patients with node-positive disease can be considered for patients whose initial treatment was tamoxifen or whose initial therapy was less than 5 years of an aromatase inhibitor.
Multigene assays might be used in the future to select patient candidates for extending adjuvant endocrine therapies. Recently, the PAM50 signature was tested on 1478 tissue samples derived from a large cohort of endocrine-responsive breast cancer enrolled in the Austrian Breast and Colorectal Cancer Study Group (ABCSG) 8 study. The estimated percentage without recurrence at 15 years assessed by PAM50 was 95.6% for low risk of recurrence, 87.3% for intermediate risk of recurrence, and 72.1% for high risk of recurrence.
Based on available data, it might be argued that luminal A cancers are resistant to chemotherapy, even if they have some adverse prognostic features, such as multiple positive nodes. As discussed previously, however, the best evidence supporting the theory of chemotherapy resistance in luminal A cancers is based on 2 retrospective, unplanned subgroup analyses of clinical trials performed some years ago.
Evidence from an Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) overview is that cytotoxic chemotherapy is, on average, beneficial in delaying relapse and prolonging survival for women with early breast cancer and, therefore, recommended for the treatment of patients with node-positive disease, irrespective of the tumor biology. It may, therefore, be concluded that because even a few patients may benefit, all should receive chemotherapy. This ignores, however, the potential harm of delaying effective endocrine therapy to those who most need it as well as the obvious harm of exposing a large majority to the toxic (occasionally fatal) adverse effects of chemotherapy.
In the few cases of luminal A disease and large tumor burden (eg, multiple positive nodes), it seems reasonable to offer chemotherapy, although benefit remains uncertain, because lymph node status is not predictive to sensitivity for either endocrine treatment or chemotherapy.
When controversies remain for deciding to give chemotherapy in ER-positive and HER2-negative cases, the use of gene array analysis might be useful for the selection of patients who might forego chemotherapy. The 21-gene RS that was demonstrated as predictive of chemotherapy responsiveness should be considered for this analysis when indicated.
If given, chemotherapy for such patients could comprise less-intensive regimens, such as Adriamycin (doxorubicin) and cyclophosphamide (AC) ×4, CMF ×6, or Taxotere (docetaxel) and cyclophosphamide (TC) ×4, that demonstrated activity in luminal disease in previous studies.
In the decision of whether to introduce adjuvant chemotherapy, the presence of special types should be taken into consideration. Little attention has been dedicated in the past to identification of special types of breast cancer, that is, those displaying a distinct morphology might exhibit a distinct prognostic and predictive profile compared with invasive ductal carcinomas of no special type or not otherwise specified.
Within luminal A breast cancer, several special types display an extremely good prognosis, often approaching or equaling that of the general population. In particular, pure tubular and cribriform carcinomas represent a rare histologic type, correlated with a favorable prognosis. Current published data indicate that, compared with grade 1 ductal carcinomas, tubular carcinoma is associated with longer disease-free survival and breast cancer–specific survival close to normal life expectancy.
In locally advanced invasive lobular carcinomas, response to primary chemotherapy seemed lower in terms of pathologic complete response (0%–3%) compared with invasive ductal carcinoma.
Lobular carcinomas are characterized by a significantly higher expression of steroid hormone receptors compared with the ductal ones, which might contribute to the lower response to preoperative chemotherapy. In addition, a large retrospective study identified 981 patients with lobular disease who had no benefit from the addition of adjuvant chemotherapy, whereas poorer disease-free survival was observed for patients with endocrine-responsive tumors who did not receive any adjuvant hormonal therapy (HR 2.35; 95% CI, 1.05–5.23).
These results indicate that a tailored approach should be considered in classical lobular carcinoma, based on proper adjuvant endocrine therapy administered for perhaps a prolonged period of time.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




