Lung cancer continues to be the leading cause of cancer-related mortality in the United States. Brain metastases are a significant problem in patients with lung cancer and have conventionally been treated with whole-brain radiation. This article reviews the data for systemic chemotherapy to treat brain metastasis from lung cancer and examines the activity of small molecule tyrosine kinase inhibitors for the targeted therapy for brain metastases from EGFR -mutant and ALK -rearranged non–small cell lung cancer. Future directions for evaluating the role of immunotherapy in treating brain metastasis are also discussed.
Key points
- •
Brain metastases are a significant problem in patients with lung cancer, with lung cancer accounting for 50% of brain metastases diagnosed on CT scans.
- •
For patients with small cell lung cancer (SCLC) and widespread systemic disease along with asymptomatic intracranial brain metastases, it is reasonable to treat upfront with systemic chemotherapy, delaying whole-brain radiation therapy (WBRT) until symptomatic progression of brain metastases.
- •
Platinum doublet agents are active in the treatment of asymptomatic brain metastases from non–SCLC (NSCLC) but do not replace radiation.
- •
For selected patients with targetable molecular alterations detected in their tumors, small molecule tyrosine kinase inhibitor (TKI) therapy may be appropriate for treatment of brain metastases.
- •
The role of immunotherapy in the treatment of brain metastases from lung cancer remains to be established.
Introduction
Lung cancer continues to be the leading cause of cancer-related death in the United States. Approximately 40% of patients have metastatic disease at presentation and are treated with palliative intent, with the goals of maintaining quality of life and improving survival. Brain metastases are a significant problem in patients with lung cancer, which accounts for 50% of brain metastases diagnosed by CT scans. With the advent of improved imaging modalities, such as MRI for staging and detection of subclinical disease, coupled with the modest improvement in survival from therapeutic advances in the systemic treatment of lung cancer, an increase in incidence of brain metastases is expected.
The histologic distribution of metastases from lung cancer is as follows: small cell carcinoma 31%, adenocarcinoma 21%, large cell carcinoma 21%, and squamous cell carcinoma 8%. It is estimated that approximately 8% of patients with NSCLC have brain metastases at presentation, with clinical risk factors, including younger age, female gender, adenocarcinoma or large cell histology, tumor size greater than 3 cm, higher tumor grade, and regional lymph node involvement. A retrospective review of patients with stage III NSCLC treated with combined modality therapy on Southwest Oncology Group protocols reveals a cumulative 24-month estimate for brain metastases of 22% for adenocarcinoma, 8% for squamous NSCLC, and 17% for other histologies.
WBRT has been demonstrated to improve outcome in patients with brain metastases with brain response rate (RR) of 35%, resulting in improvement in headache (82%), lost motor function (61%–74%), and mentation. The Radiation Therapy Cooperative Group (RTOG) 0901 and 7361 studies demonstrated that there was no significant difference in survival with different fractionation schedules for palliative WBRT, with reported median overall survival (OS) of 4.1 and 3.4 months in these 2 studies, respectively. Furthermore, the RTOG 7606 found no improvement in survival with a dose of 50 Gy compared with the standard 30-Gy dose. The RTOG 9104 compared 32 Gy twice daily radiation to 30 Gy in 10 fractions and also found no survival advantage for the higher dose of 32 Gy twice daily. Therefore, 30 Gy over 10 fractions is the standard WBRT regimen.
Several prognostic factors in patients with brain metastases have been described. Gaspar and colleagues examined a 1200-patient data set pooled from 3 RTOG trials conducted between 1979 and 1993. They used recursive partitioning analysis, a statistical tool used to create a regression tree according to prognostic indicators, which divided patients into 3 classes. The best survival was noted in patients less than 65 years of age with Karnofsky performance scale score (KPS) of 70 or more and controlled primary tumor with brain as only site of metastases, with median survival of 7.1 months (class I). The worst survival was seen in patients with KPS less than 70, with median survival of 2.3 months (class III), whereas the remaining patients (class II) had a median survival of 4.2 months.
The graded prognostic index is another model developed using 4200 patients from 5 RTOG studies. For patients with lung cancer, 4 variables were included in the model: age, KPS, number of metastases, and presence of extracranial metastases, based on which scores were assigned to each variable. The cumulative scores were used to determine prognosis: a score of 3.5 to 4 was associated with a median survival of 11 months, whereas at the other extreme, a score of 0 to 1 was associated with a median survival of 2.8 months.
For select patients with limited number of metastases, stereotactic brain radiosurgery (SRS) may be used. In the RTOG 9005 study, 156 patients with recurrent brain tumors were treated with SRS. Tumors less than 2 cm were treated to 24 Gy and tumors larger than 2 cm up to 3 cm were treated to 18 Gy whereas tumors between 3.1 cm and 4 cm were treated to 15 Gy. The median survival was 11.4 months. Prognosis varies according to number of lesions, largest lesion volume, age, KPS, and status of systemic disease and a Score Index for Radiosurgery has been developed ranging from 0 to 10. Patients with score 1 to 2 have median survival of 2.9 months whereas those with 8 to 10 have survival of 31.4 months.
Introduction
Lung cancer continues to be the leading cause of cancer-related death in the United States. Approximately 40% of patients have metastatic disease at presentation and are treated with palliative intent, with the goals of maintaining quality of life and improving survival. Brain metastases are a significant problem in patients with lung cancer, which accounts for 50% of brain metastases diagnosed by CT scans. With the advent of improved imaging modalities, such as MRI for staging and detection of subclinical disease, coupled with the modest improvement in survival from therapeutic advances in the systemic treatment of lung cancer, an increase in incidence of brain metastases is expected.
The histologic distribution of metastases from lung cancer is as follows: small cell carcinoma 31%, adenocarcinoma 21%, large cell carcinoma 21%, and squamous cell carcinoma 8%. It is estimated that approximately 8% of patients with NSCLC have brain metastases at presentation, with clinical risk factors, including younger age, female gender, adenocarcinoma or large cell histology, tumor size greater than 3 cm, higher tumor grade, and regional lymph node involvement. A retrospective review of patients with stage III NSCLC treated with combined modality therapy on Southwest Oncology Group protocols reveals a cumulative 24-month estimate for brain metastases of 22% for adenocarcinoma, 8% for squamous NSCLC, and 17% for other histologies.
WBRT has been demonstrated to improve outcome in patients with brain metastases with brain response rate (RR) of 35%, resulting in improvement in headache (82%), lost motor function (61%–74%), and mentation. The Radiation Therapy Cooperative Group (RTOG) 0901 and 7361 studies demonstrated that there was no significant difference in survival with different fractionation schedules for palliative WBRT, with reported median overall survival (OS) of 4.1 and 3.4 months in these 2 studies, respectively. Furthermore, the RTOG 7606 found no improvement in survival with a dose of 50 Gy compared with the standard 30-Gy dose. The RTOG 9104 compared 32 Gy twice daily radiation to 30 Gy in 10 fractions and also found no survival advantage for the higher dose of 32 Gy twice daily. Therefore, 30 Gy over 10 fractions is the standard WBRT regimen.
Several prognostic factors in patients with brain metastases have been described. Gaspar and colleagues examined a 1200-patient data set pooled from 3 RTOG trials conducted between 1979 and 1993. They used recursive partitioning analysis, a statistical tool used to create a regression tree according to prognostic indicators, which divided patients into 3 classes. The best survival was noted in patients less than 65 years of age with Karnofsky performance scale score (KPS) of 70 or more and controlled primary tumor with brain as only site of metastases, with median survival of 7.1 months (class I). The worst survival was seen in patients with KPS less than 70, with median survival of 2.3 months (class III), whereas the remaining patients (class II) had a median survival of 4.2 months.
The graded prognostic index is another model developed using 4200 patients from 5 RTOG studies. For patients with lung cancer, 4 variables were included in the model: age, KPS, number of metastases, and presence of extracranial metastases, based on which scores were assigned to each variable. The cumulative scores were used to determine prognosis: a score of 3.5 to 4 was associated with a median survival of 11 months, whereas at the other extreme, a score of 0 to 1 was associated with a median survival of 2.8 months.
For select patients with limited number of metastases, stereotactic brain radiosurgery (SRS) may be used. In the RTOG 9005 study, 156 patients with recurrent brain tumors were treated with SRS. Tumors less than 2 cm were treated to 24 Gy and tumors larger than 2 cm up to 3 cm were treated to 18 Gy whereas tumors between 3.1 cm and 4 cm were treated to 15 Gy. The median survival was 11.4 months. Prognosis varies according to number of lesions, largest lesion volume, age, KPS, and status of systemic disease and a Score Index for Radiosurgery has been developed ranging from 0 to 10. Patients with score 1 to 2 have median survival of 2.9 months whereas those with 8 to 10 have survival of 31.4 months.
Systemic chemotherapy for small cell lung cancer
Brain metastases from SCLC have been conventionally treated with WBRT. Given the frequency of brain metastases from SCLC and poor prognosis associated with them, prophylactic cranial radiation is recommended for patients with limited stage disease. The thought had been that chemotherapeutic agents do not cross the blood-brain barrier in sufficient concentrations to result in an intracranial response. Nevertheless, disruption of the blood-brain barrier by metastases can actually allow intracranial penetration of systemic agents. This is further supported by early reports of intracranial response in patients with SCLC treated with combination systemic chemotherapy.
Front-Line Platinum-based or Cyclophosphamide-Based Regimens
A small prospective study using a regimen of etoposide, cyclophosphamide, doxorubicin, and vincristine in 14 patients with previously untreated SCLC with asymptomatic brain metastases reported an intracranial RR of 82%, with no deterioration of neurologic status ( Table 1 ).
| Regimen | N | Study Type | Prior Whole-brain Radiation Therapy | Prior Chemotherapy | Brain Response Rate (%) | Median Survival (mo) | Reference |
|---|---|---|---|---|---|---|---|
| Cyclophosphamide, doxorubicin, vincristine and etoposide with WBRT | 14 | Single-arm prospective study, WBRT added at cycle 4 | No | No | 82 | 7.8 | Lee et al, 1989 |
| Cyclophosphamide, vincristine, etoposide | 19 | Retrospective analysis of patients with brain metastasis in 610-patient randomized trial | No | No | 53 | 6.5 | Twelves et al, 1990 |
| Cyclophosphamide, doxorubicin, and etoposide | 24 | Prospective single-arm 181-patient study that allowed asymptomatic brain metastases, received WBRT if became symptomatic | No | No | 27 | 8.3 | Seute et al, 2006 |
| Cisplatin, teniposide, vincristine, followed by multidrug regimen | 21 | Prospective study, brain radiation withheld till progression in brain | No | No | 85 | 3.6 | Kristjansen et al, 1993 |
| Carboplatin and irinotecan | 15 | Phase II | Yes, 13/15 | Yes, all patients | 65 | 6 | Chen et al, 2008 |
| Carboplatin | 19 | Symptomatic brain metastases | 40 | 3.5 | Groen et al, 1993 | ||
| Topotecan | 30 | Phase II | Yes, 8/30 | Yes, all patients | 33 | 3.6 | Korfel et al, 2002 |
| Teniposide with or without WBRT | 120 | Phase III randomized study | No | No | 57 in combination arm, 22 in teniposide-only arm | 3.5 vs 3.2 | Postmus et al, 2000 |
The current standard of care for the front-line treatment of SCLC includes a platinum agent with either etoposide or irinotecan. The intracranial RR to single-agent carboplatin is 40% in patients with symptomatic brain metastasis, whereas it is higher for the carboplatin and irinotecan combination at 65%. These data suggest that for patients with widespread systemic disease along with asymptomatic intracranial brain metastases, it is reasonable to initiate the treatment with systemic chemotherapy while delaying WBRT until the completion of the initial treatment course or progression of the brain metastases.
Topotecan
Topotecan is the only agent approved in the second-line setting for relapsed SCLC. A phase II study of this agent was conducted in 30 patients with relapsed SCLC with asymptomatic brain metastases and 14 of them had received 1 prior line of chemotherapy, whereas 16 had received at least 2 prior lines of treatment and 8 patients had received prior WBRT. The first 22 patients received the planned dose of topotecan, 1.5 mg/m 2 , intravenously as a 30-minute infusion for 5 consecutive days of a 21-day cycle, but due to thrombocytopenia, the last 8 patients were treated with the lower dose of 1.25 mg/m 2 . Intracranial response was observed in 33% of the treated patients, which was numerically higher than the systemic RR of 29%. Myelosuppression is a common toxicity of this agent, however, with grade 3 and grade 4 leukopenia observed in 28% and 22% of patients respectively. Grade 3 and grade 4 thrombocytopenia were observed in 17% and 11% of patients, respectively, and 17% of patients had grade 3 infection.
Systemic chemotherapy for non–small cell lung cancer
Brain metastases are a significant problem in patients with NSCLC, with approximately 8% requiring brain radiotherapy at presentation. Unlike SCLC, PCI has not been shown to improve median survival in patients with stage IIII NSCLC. Several studies have evaluated the role of chemotherapy in patients with brain metastases from NSCLC ( Table 2 ).
| Regimen | N | Study Type | Brain Response Rate (%) | Median Survival (mo) | Reference |
|---|---|---|---|---|---|
| Carboplatin and paclitaxel | 5 | Preliminary data from prospective study | 20 | — | Lee et al, 1997 |
| Cisplatin and vinorelbine with early vs delayed WBRT | 176 | Phase III | 33 for early WBRT, 27 for delayed | 5.1 for early WBRT vs 5.5 for delayed | Robinet et al, 2001 |
| Cisplatin and pemetrexed | 43 | Phase II | 41.9 | 7.4 | Barlesi et al, 2011 |
| Cisplatin and pemetrexed with concurrent WBRT | 42 | Phase II | 68.3 | 12.6 | Dinglin et al, 2013 |
Platinum Agents
Historically, trials of first-line chemotherapy in NSCLC have excluded patients with brain metastases due to their poor prognosis. Only 1 patient had intracranial response in a preliminary report of 5 patients treated on a clinical trial of carboplatin and paclitaxel, for an intracranial RR of 20%. The phase III Groupe Français de Pneumo-Cencérologie (GFPC) protocol 95-1 compared early versus delayed WBRT with concurrent cisplatin and vinorelbine in 176 patients with inoperable brain metastases. The intracranial RRs were 27% for the delayed radiation (chemotherapy-alone) group and 33% for the chemotherapy with early WBRT. The median survival duration was not significantly different between the 2 groups (24 and 21 weeks respectively, P = .83). These results suggested that timing of WBRT did not influence survival of NSCLC with brain metastases when treated with concurrent chemotherapy. Two phase II studies evaluated the activity of cisplatin and pemetrexed in the treatment of brain metastasis. The GFPC 07-01 study enrolled chemotherapy-naïve patients with metastatic NSCLC and brain metastases who were ineligible for radiosurgery to up to 6 cycles of cisplatin, 75 mg/m 2 , and pemetrexed, 500 mg/m 2 , every 3 weeks. Patients received WBRT at the time of chemotherapy completion or at disease progression. The brain metastases RR was 41.9%, whereas extracranial RR was 34.9%. The median survival time in the study was 7.4 months. In a second phase II trial, 42 patients with metastatic NSCLC to the brain were treated with up to 6 cycles of the same regimen of cisplatin and pemetrexed concurrent with WBRT to a dose of 30 Gy in 10 fractions. The RR in the brain and extracranially were 68.3% and 34.1%, respectively, with a median OS of 12.6 months.
Temozolomide
Temozolomide is an alkylating agent approved for the treatment of primary brain tumors, such as glioblastoma. Its intracranial activity led to the study of this agent’s activity in tumors that are metastatic to the brain. Two phase II clinical trials evaluated the activity of single-agent temozolomide in heavily pretreated patients with solid tumors, including NSCLC and SCLC, with brain metastases. Both studies used a regimen of temozolomide, 150 mg/m 2 /d, for 5 days, repeated every 28 days and reported disease control rates (DCRs) of 20.8% to 41% with a median OS of 4.5 to 6.6 months. In the larger of the two studies, which enrolled 41 patients and including 22 with NSCLC, 2 patients (9%) had a partial response (PR). A second study investigated temozolomide in 30 patients with NSCLC with progression of brain metastases after at least 1 line of chemotherapy and WBRT. The dose of temozolomide was escalated to 200 mg/m 2 /d with subsequent cycles if no grade 3 or grade 4 hematologic toxicities were observed. Three patients (10%) achieved objective response of brain metastases, with 2 complete responses (CRs). No grade 3 or grade 4 toxicities were observed. Three patients were long-term survivors, having survived beyond a year after the start of temozolomide.
The European Organisation for Research and Treatment of Cancer Lung Cancer Group 08965 study evaluated the activity of single-agent temozolomide, 200 mg/m 2 /d for 5 days every 28 days, in chemotherapy-naïve patients with NSCLC. The study included 2 groups of patients, those with brain metastases (n = 12) and patients without brain metastases (n = 13). Treatment was continued until disease progression or unacceptable toxicity, for a maximum of 6 cycles. This study was closed early due to lack of objective responses in either group of patients.
Temozolomide has also been studied in combination with brain radiotherapy. The RTOG 0320 trial was a phase III trial of WBRT at a dose of 37.5 Gy in 15 fractions and SRS alone versus WBRT and SRS with either temozolomide or erlotinib in patients with 1 to 3 brain metastases. The primary endpoint was OS. This study closed prematurely due to poor accrual after enrolling 126 patients. The median survival times for WBRT and SRS–alone group of 13.4 months was numerically higher (although not statistically significant) than WBRT and SRS with temozolomide (6.3 months) or WBRT, SRS, and erlotinib (6.1 months), suggesting a trend toward possible deleterious effect of adding temozolomide or erlotinib to WBRT and SRS.
Antifolate Agents
Pemetrexed is an antifolate chemotherapeutic agent approved for the treatment of nonsquamous NSCLC. In patients with central nervous system (CNS) metastases, pemetrexed distributes from the plasma to the cerebrospinal fluid (CSF) within 1 to 4 hours of dosing, and the resulting CSF concentration of pemetrexed is less than 5% of the plasma concentration. A total of 21 patients with CNS metastases were treated with intravenous pemetrexed at doses of 500 mg/m 2 (n = 3), 750 mg/m 2 (n = 3), 900 mg/m 2 (n = 12), or 1050 mg/m 2 (n = 3) every 3 weeks with restaging neuroimaging performed every 6 weeks in a small study. This study included 4 patients with metastatic lung cancer, 3 of whom had received 1 prior line of chemotherapy, and the fourth patient had received 2 prior lines of chemotherapy. Three of the 4 patients had received prior WBRT. No intracranial responses were observed in patients with lung cancer, although 2 patients had stable disease.
Intrathecal Chemotherapy
Leptomeningeal carcinomatosis is associated with significant morbidity and poor outcome. Intrathecal (IT) chemotherapy, mostly with methotrexate and cytarabine, has been used as a treatment modality in this setting. A randomized controlled trial of IT sustained-release cytarabine (DepoCyt) versus IT methotrexate in 61 patients with solid tumors and positive CNS cytology included 3 patients with NSCLC and 2 patients with SCLC. Patients were randomized to IT DepoCyt (31 patients) or IT methotrexate. The RRs were similar among the DepoCyt group (26%) and methotrexate group (20%), but time to neurologic progression was greater in the DepoCyt arm compared with the methotrexate arm (58 days vs 30 days; log-rank test P = .007).
Molecularly targeted therapy for brain metastasis
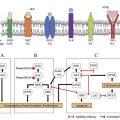
Lung cancer is a genetically complex disease and the study of molecular mechanisms leading to brain metastases in this malignancy has been particularly challenging, because often patients are treated for their brain lesions based on radiographic findings. In select cases, where surgical resection is clinically indicated, tissue suitable for molecular analysis can be obtained. The paired analysis of patient-matched primary and brain metastases, from cancers including lung, has shed some light on the branched evolution of brain metastases relative to the primary tumors, and potential targets for cyclin dependent kinase inhibitors, inhibitors of EGFR, phosphatidyl inositol 3 kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR), and mitogen-activated protein kinase (MAPK) cyclin-dependent kinase (CDK) pathways.
The identification of targetable somatic molecular alterations, such as epidermal growth factor receptor ( EGFR ) mutations and anaplastic lymphoma kinase ( ALK ) gene rearrangements, has changed standard of care in select patients.
Epidermal Growth Factor Receptor
EGFR mutations have been reported in 22% of patients with metastatic lung adenocarcinoma enrolled in the Lung Cancer Mutation Consortium study. EGFR mutations are seen more commonly in patients who are never-smokers or light smokers, of Asian ethnicity, and are female gender. The first-generation reversible EGFR-TKIs, erlotinib and gefitinib, have demonstrated improved progression-free survival (PFS) compared with front-line chemotherapy in patients with EGFR -mutant NSCLC, which led to their approval by the Food and Drug Administration (FDA) for the treatment of patients with metastatic EGFR -mutant NSCLC. Case reports and case series emerged of impressive intracranial responses in patients treated with EGFR-TKI therapy. This led to several retrospective and prospective studies evaluating the intracranial response to EGFR-TKI therapy, either alone or in combination with brain radiotherapy ( Table 3 ). Intracranial RRs range from 58.3% in Asian patients with unselected adenocarcinoma and asymptomatic brain metastases, which included some EGFR -mutant lung cancers, to as high as 88.9% in patients with EGFR -mutant NSCLC.
| Classification | Agent | Activating Epidermal Growth Factor Receptor Mutation | Study Type | N | Brain Response Rate (%) | Median Overall Survival (mo) | Reference |
|---|---|---|---|---|---|---|---|
| First generation | Gefitinib | Yes | Retrospective | 9 | 88.9 | — | Li et al, 2011 |
| Gefitinib | Yes | Phase II clinical trial | 41 | 87.8 | 21.9 | Iuchi et al, 2013 | |
| Erlotinib | Yes | Retrospective | 17 | 82.4 | 12.9 | Porta et al, 2011 | |
| Erlotinib | Asian, adenocarcinoma, included some EGFR-mutant | Phase II clinical trial, asymptomatic brain metastases | 48 | 58.3 | 18.9 | Wu et al, 2013 | |
| Erlotinib plus concurrent WBRT | Unselected | Phase II clinical trial | 40 | 86 | 11.8 | Welsh et al, 2013 | |
| WBRT with or without erlotinib | Unselected | Phase II, if mutation status known, received WBRT with erlotinib, if unknown, received WBRT monotherapy | 54; 23 got erlotinib | 95.6 | 10.7 | Zhuang et al, 2013 | |
| High-dose pulsatile weekly erlotinib | Yes | Case series | 9 | 67 | 12 | Grommes et al, 2011 | |
| Gefitinib or erlotinib | Yes | Phase II clinical trial | 28 | 83 | 15.9 | Park et al, 2012 | |
| Gefitinib or erlotinib | Asian never-smokers | Retrospective | 23 | 73.9 | 18.8 | Kim et al, 2009 | |
| Second generation | Afatinib | Yes | TKI pretreated, as part of compassionate use program | 31 | 35 | 9.8 | Hoffknecht et al, 2015 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree