Insights into the biology of clear-cell renal cell carcinoma (CCRCC) have identified multiple pathways associated with the pathogenesis and progression of this cancer. This progress has led to the development of multiple agents targeting these pathways, including the tyrosine kinase inhibitors sorafenib, sunitinib, and pazopanib, the monoclonal antibody bevacizumab, and the mTOR inhibitors temsirolimus and everolimus. With the exception of temsirolimus, phase 3 trials tested these agents in patients with clear-cell histology; therefore, their efficacy in non-CCRCC is unclear. To date, there is no established effective therapy for patients with advanced non-CCRCC. This article focuses on treatment options for metastatic non-CCRCC.
Renal cell carcinoma (RCC) affects more than 40,000 patients in the United States each year. Localized disease is curable with surgery, but a significant proportion of patients relapse or present with metastatic disease that is largely incurable. Until relatively recently all adult renal epithelial tumors were labeled as “renal cell carcinomas” or “kidney cancers.” Over the last 15 years RCC has increasingly been recognized as a heterogeneous disease with several distinct subtypes that have differing clinical, pathologic, and molecular characteristics.
RCCs can be divided into clear-cell (CCRCC, 70%–80%), and non–clear-cell (NCCRCC) histologies. The latter mainly include papillary (PRCC, 10%–15%), chromophobe (ChRCC, 5%), unclassified (5%), collecting duct, and medullary (CDRCC, MRCC, <5%). In the era of immunotherapy, metastatic CCRCC was perceived to have a better outcome than PRCC, but this has been contradicted by a large study of 1001 patients with metastatic RCC (82 of which had PRCC), showing similar 5-year survival rates of around 10% irrespective of clear-cell or papillary histology. ChRCC is acknowledged to have the best overall prognosis compared with other subtypes, in both local and metastatic disease, and the same study confirmed this, indicating 5-year survival rates of 87.9% in ChRCC compared with 73.2% in CCRCC.
In the past decade, various targeted therapies such as tyrosine kinase inhibitors (TKIs), mammalian target of rapamycin (mTOR) inhibitors, and vascular endothelial growth factor (VEGF) monoclonal antibodies have changed the paradigm of CCRCC management. However, a key unresolved issue is whether these therapies can replicate their efficacy in NCCRCC. Indeed, most clinical trials to date have focused on patients with clear-cell histology. Retrospective analysis of these trials has indicated potential activity of targeted agents in NCCRCC and, as such, prospective trials have been initiated. This review outlines the different subtypes of NCCRCC, as well as the latest therapeutic developments in NCCRCC.
Development of targeted agents
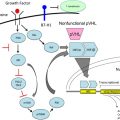
Improved understanding of the molecular biology underlying RCC has led to the development of several drugs that specifically target distinct pathways, and there is now convincing evidence that they are of benefit in patients with clear-cell histology. This evidence raises the question of whether VEGF is a valid target in NCCRCC. Despite the fact that VHL inactivation and the subsequent overexpression of hypoxia-inducible genes such as VEGF are hallmarks of CCRCC, patients with papillary, chromophobe, and medullary histology can still demonstrate high expression of VEGF, VEGF receptor 1 (VEGFR1), and VEGFR2 (especially in more advanced stages) that is correlated with worse survival, making VEGF-targeted therapy an attractive therapeutic option. There are currently two major classes of targeted agents of particular interest for treatment of NCCRCC.
Tyrosine Kinase Inhibitors
Kinase inhibitors are drugs that generally inhibit tyrosine kinase (TK) enzymes, which catalyze the transfer of phosphate groups from adenosine triphosphate (ATP) to tyrosine residues on proteins. This process can be an activating event for proteins involved in signaling, and leads to increased cellular proliferation and the promotion of angiogenesis and metastasis. Receptor tyrosine kinases (RTKs) such as the epidermal growth factor receptor (EGFR) are located in the cell membrane and transduce signals from the extracellular environment to the cell interior. Numerous downstream signaling pathways such as RAS/RAF/MEK/ERK and PI3K (phosphoinositol 3′-kinase)/Akt may be activated by ligand binding to a RTK. Nonreceptor tyrosine kinases such as c-ABL are located intracellularly and can be activated by mechanisms such as phosphorylation. TKIs disrupt TK signaling by preventing the binding of either protein substrates or ATP, and examples of TKIs with activity in NCCRCC include sunitinib, sorafenib, erlotinib, and pazopanib.
mTOR Inhibitors
mTOR is a nonreceptor serine/threonine kinase in the PI3K/Akt pathway that controls the translation of specific messenger RNA; mTOR activation has multiple downstream effects including increasing HIF-1α gene expression. Furthermore, reduced PTEN expression has been demonstrated in some renal cell carcinomas, and loss of PTEN function results in Akt phosphorylation with downstream effects on cell growth and proliferation that may be blocked using rapamycin derivatives. There is therefore a strong rationale for using mTOR inhibitors in RCC.
Sporadic papillary RCC
Pathology and Molecular Biology
Sporadic PRCC is itself a heterogeneous entity with at least 2 and possibly 3 distinct subtypes, both at the morphologic and genetic levels, which appear to have different clinical characteristics. As might be expected, most of these tumors have a papillary, tubular, or tubulopapillary growth pattern.
From a histologic standpoint, two different subtypes of PRCC are identified, type 1 with small cells and pale cytoplasm and type 2 with large cells and eosinophilic cytoplasm. Similarly, these two subtypes have distinct cytogenetic and molecular profiles that distinguish them from other renal epithelial tumors. Although only about 10% of sporadic type I PRCC have been reported to show somatic mutations in the c-MET gene, a genetic abnormality commonly seen as a germline mutation in hereditary cases, the c- Met pathway can be activated in many sporadic PRCC in the absence of c- Met mutation. The group from the National Institutes of Health described the genetic abnormality associated with the hereditary form of the type 2 papillary RCC, consisting of mutations in the fumarate hydratase ( FH ) gene. The contribution of this mutation to the pathogenesis of sporadic papillary type 2 RCC remains unknown.
More recently, Yang and colleagues proposed a refinement of the former (type I/type II) classification and introduced a molecular classification. Using gene expression profiling, they identified two highly distinct molecular PRCC subclasses with morphologic correlation. The first class, with excellent survival, corresponded to 3 histologic subtypes: type 1, low-grade type 2, and mixed type 1/low-grade type 2 tumors. The second class, with poor survival, corresponded to high-grade type 2 tumors. Dysregulation of G1-S and G2-M checkpoint genes were found in class 1 and 2 tumors, respectively. c-Met was differentially expressed, with higher expression in class 1 tumors. This refined classification of PRCC based on morphologic and molecular characteristics may be more relevant and is likely to aid diagnosis, prognosis, treatment, and analysis of clinical trials for advanced PRCC.
Treatment
Sunitinib inhibits the RTKs VEGFR2, platelet-derived growth factor receptor (PDGFR), FLT-3, and c-KIT ( Table 1 ). A dose of 50 mg orally once a day for 4 weeks followed by a 2-week break was the recommended phase 2 dose based on two phase 1 studies. It has subsequently been shown to significantly increase progression-free survival (PFS) in patients with metastatic CCRCC and has become a first-line standard of care for these patients.
| Agent | Target |
|---|---|
| Sorafenib | VEGFR2, VEGFR3, PDGFR, FLT-3, c-KIT, CRAF, wtBRAF, V600E BRAF |
| Sunitinib | VEGFR2, PDGFR, FLT-3, c-KIT |
| Temsirolimus | mTOR |
| Erlotinib | EGFR |
| Foretinib (GSK1363089) (previously XL880) | MET, VEGFR2 |
A worldwide expanded access trial of sunitinib has been undertaken, with a primary purpose to make the drug available to patients before regulatory approval. More than 4000 patients have been enrolled into this study, giving an important database especially for subgroup analysis. In May 2007, Gore and colleagues presented data on 2341 patients, the majority of whom (78%) had received prior cytokine therapy. A subgroup analysis of patients with non–clear-cell histology was performed and 276 patients (11.8%) with non–clear-cell histology were identified, although distinction between different subtypes was not made. A response rate of 5.4%, clinical benefit (defined as response and stable disease >3 months) of 47% and median PFS of 6.7 months was seen in this subgroup. This result compared with an overall response rate for the entire patient group of 9.3%, clinical benefit of 52.3%, and median PFS of 8.9 months. The investigators concluded that sunitinib was active in the non–clear-cell subgroup; however, these data need to be interpreted with caution because of the nonrandomization of patients in the expanded access trial and the lack of pathology verification.
In light of the results of the retrospective subgroup analysis, further trials have been initiated to provide additional data on sunitinib activity in NCCRCC. In 2008, Plimack and colleagues reported preliminary results from a phase 2 study of sunitinib in patients with NCCRCC. In a cohort of 26 patients of whom 13 had PRCC there were no objective responses, although 8 patients did experience stable disease. Moreover, the response rate and median PFS (48 days) were disappointing. Recently, updated results from this trial have been reported. The trial has been expanded to include 48 patients, with analysis focused on the patients with PRCC (23). Unfortunately, the results remained disappointing; among the PRCC patients the median PFS was 1.6 months (95% confidence interval [CI] 1.3–12), the median overall survival (OS) was 10.8 months (95% CI, 6.2 to not evaluable), and no major responses were observed, with the best response being stable disease (seen in 8 patients).
The SUPAP study is another phase 2 trial investigating sunitinib activity in type 1 and 2 PRCC. Twenty-eight patients were enrolled, and of the 23 patients with type 2 PRCC, 1 had a partial response and 13 had stable disease (lasting for ≥12 weeks in 4 patients). Five patients had type 1 PRCC, and although none experienced a partial response, 3 had stable disease. Based on these results, the investigators concluded that sunitinib did have some activity in PRCC, albeit inferior compared with CCRCC.
These conclusions have been supported by the results of another phase 2 study conducted in a cohort of 23 NCCRCC patients by Molina and colleagues. There were 8 patients with PRCC, and in this subgroup no partial responses were seen, with a median PFS of 5.6 months (95% CI 1.4–7.1). The data from recent phase 2 studies has therefore tempered the initial optimism raised by the retrospective subgroup analysis, and it appears that sunitinib at best has modest activity in PRCC. Nevertheless, there are still several ongoing phase 2 trials investigating sunitinib therapy for PRCC, and their results will be useful in clarifying the role of sunitinib in NCCRCC ( Clinicaltrials.gov identifier NCT00465179, NCT01034878, and NCT01219751). One study of 9 patients from Korea was preliminarily presented at the 2011 Genitourinary Cancers Symposium, and showed a response rate of 38% and a time to progression of 6.4 months. The investigators considered the primary end point has been met, and suggested that sunitinib has promising activity in patients with NCCRCC.
Sorafenib inhibits the RTKs VEGFR2, VEGFR3, FLT-3, c-KIT, and PDGFR, and the nonreceptor serine threonine kinases BRAF and CRAF (see Table 1 ). The BRAF and CRAF kinases are members of the RAF/MEK/ERK signaling cascade, which is involved in the survival and proliferation of tumor cells and is a therapeutic target in cancer, although it is not known to be of major importance in RCC. Sorafenib has subsequently been shown to significantly increase PFS in patients with metastatic CCRCC who had progressed on cytokine therapy, and is licensed for the treatment of metastatic RCC.
Ratain and colleagues were among the first to administer sorafenib for metastatic PRCC. In a phase 2 randomized discontinuation study; they treated 15 PRCC patients out of a total of 202 patients. From this subgroup, 2 patients achieved a partial response and 3 had tumor shrinkage of 25% to 49%; this was comparable to the entire population and indicated sorafenib activity in PRCC.
In one of the largest detailed series to date, Choueiri and colleagues reported on the efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe RCC. This retrospective analysis identified 53 patients who had been treated with either sunitinib or sorafenib at 5 different cancer centers in the United States and France. In contrast to the expanded access studies, expert genitourinary pathologists from each institution reviewed the cases to confirm the histopathological diagnosis of NCCRCC. Forty-one patients had PRCC; 13 were treated with sunitinib and of these, 2 patients achieved a partial response (15% response rate), with durations of 12 months and more than 8 months. No responses were seen in the 28 patients treated with sorafenib. In total, 27 patients (68%) achieved stable disease for more than 3 months after 2 cycles of treatment with sunitinib or sorafenib. Minor responses ranging from −4% to −25% were seen in 9 patients. PRCC patients had a PFS of 7.6 months, and it was observed that treatment with sunitinib resulted in a superior PFS compared with sorafenib (PFS 11.9 vs 5.1 months, respectively; P <.001), and this remained statistically significant even after adjusting for other important prognostic factors in metastatic RCC such as hemoglobin and the number of metastatic sites.
A worldwide expanded access trial of sorafenib has also been undertaken. Response data on the Advanced Renal Cell Carcinoma Sorafenib (ARCCS) expanded access trial in North America has recently been reported on 1891 patients out of a total of 2504 patients enrolled. This study contained a subgroup of 107 PRCC patients with valid data. Within this subgroup, 3 patients (3%) exhibited partial responses, with 87 patients (81%) experiencing stable disease lasting for at least 8 weeks. This study also included an extension protocol for which NCCRCC patients and patients who had not received prior therapy were eligible, although specific distinctions between NCCRCC subtypes were not made. Data were available for 248 patients in this extension protocol; NCCRCC patients (n = 26) had a PFS of 46 weeks (95% CI 30–59; censorship rate 39%) compared with first-line patients who had a PFS of 36 weeks (95% CI 33–45; censorship rate 56%). Overall in the whole trial, toxicities for NCCRCC patients did not differ from those seen in patients with CCRCC, and sorafenib was well tolerated in both groups. Moreover, it was concluded that sorafenib appeared to have activity against PRCC.
A similar European expanded access study of sorafenib was undertaken (the European ARCCS). This study included 118 patients with PRCC of whom 104 were evaluable for response. The disease control rate was 66.4% and the median PFS was 5.8 months for PRCC compared with 75.7% and 7.5 months for patients with CCRCC, respectively.
Overall, currently available data from retrospective and expanded access studies suggest that sorafenib may possess activity against PRCC. Smaller-scale studies have also supported this impression. Unnithan and colleagues investigated cell lines established from primary and metastatic tumors from a patient with type II PRCC, and reported that sorafenib inhibited cell growth and expression of angiogenic genes such as VEGF and PDGF. Given its apparent promising activity, further trials may be necessary to confirm whether sorafenib is suitable for NCCRCC therapy.
Temsirolimus, a derivative of sirolimus (rapamycin), inhibits mTOR (see Table 1 ). Temsirolimus has been studied in a 3-arm phase 3 study comparing temsirolimus, interferon-α (IFN-α), and the combination of the two agents as first-line therapy for poor-risk patients with metastatic RCC. Response rates were similar in all 3 arms and ranged between 7% and 11%, but median OS was longer in the temsirolimus single-agent arm in comparison with the other 2 arms (10.9 months for temsirolimus, 7.3 months for IFN-α, and 8.4 months for the combination; hazard ratio [HR] 0.73, P = .0069 for single-agent temsirolimus). The investigators concluded that temsirolimus as a single agent significantly improves OS of patients with metastatic RCC and poor-risk features as compared with IFN-α, but the combination of the two drugs does not improve OS.
In this study, approximately 20% of all patients had non–clear-cell histology. Of these patients, 75% had PRCC. A subset analysis has been performed to determine the effect of temsirolimus versus IFN-α on OS and PFS in patients with clear-cell or other histologies. For NCCRCC patients (n = 73), those in the temsirolimus group had a longer OS and PFS than those in the IFN-α group (median OS 11.6 vs 4.3 months, respectively; HR 0.49; median PFS 7.0 vs 1.8 months, respectively; HR 0.38). Thus, it seems that temsirolimus may benefit patients irrespective of histology and warrants further study in patients with non–clear-cell histologies. Unfortunately, this study had no central review of the histology and therefore there was no detailed differentiation between different non–clear-cell subtypes.
More recently, Yang and colleagues performed further retrospective analysis, focusing on quality of life data gathered using the EuroQoL-5D utility score (EQ-5D index) and EQ-5D visual analog scale (EQ-VAS). It was observed that the mean EQ-5D score was higher in the temsirolimus arm compared with the IFN-α arm in NCCRCC patients.
The possibility that mTOR inhibitors have clinical activity regardless of RCC histology has led to the development of studies aimed at patients with non–clear-cell histology, and a phase 2 trial comparing temsirolimus against sunitinib as first-line therapies is currently recruiting (NCT00979966). Everolimus is another mTOR inhibitor that is being investigated by several trials. Most notably, the RAPTOR study aims to evaluate everolimus as a first-line therapy for PRCC (NCT00688753). Other ongoing trials are also investigating the use of everolimus alone, or in comparison with sunitinib, for treatment of NCCRCC (NCT00830895, NCT01185366, NCT01108445). The randomized phase 2 studies comparing mTOR inhibitors with sunitinib may help to clarify the relative role of each agent in NCCRCC.
The rationale for the use of erlotinib, an oral EGFR TKI, in PRCC stems from a study by Perera and colleagues. These investigators demonstrated that blockade of the EGFR by an anti-EGFR monoclonal antibody resulted in significant growth inhibition in NCCRCC-derived cell lines, suggesting that EGFR blockade may provide a potential therapeutic approach. In a study led by the Southwest Oncology Group (SWOG), Gordon and colleagues treated 45 patients with PRCC with erlotinib (150 mg/d). Five patients achieved a partial response for an overall response rate of 11% (95% CI 3–24) with a disease control rate (DCR) of 64% (5 partial response + 24 stable). Median OS time was 27 months (95% CI 13–36). There was no correlation between EGFR expression and disease outcome, and the drug was generally well tolerated. Although the RECIST response rate of 11% did not exceed prespecified estimates (≥20% response rate) for further study, single-agent erlotinib yielded encouraging DCR and OS results. As a result of its promising activity, two phase 2 trials are now under way to investigate erlotinib alone and in combination with bevacizumab in patients with PRCC (NCT01130519, NCT00060307).
Foretinib (GSK1363089) is a novel inhibitor of RTKs targeting MET and VEGFR. In a phase 1 study partial responses were noted in 2 out of 4 patients with PRCC, lasting for longer than 48 and 12 months. This finding has led to the initiation of a multicenter phase 2 study of foretinib (240 mg/d orally for 5 days on/9 days off) in patients with histologically confirmed PRCC. After enrollment, patients were stratified into two strata based on the presence or absence of a genetic aberration in c-MET (A: evidence of c-MET pathway activation; B: without evidence of activation). Thirty-one patients were enrolled (15 strata A and 16 strata B), and of 25 evaluable patients, 24 had at least stable disease and 20 had decreases in tumor size (range 4%–35%). Two patients had confirmed partial response and 2 had unconfirmed partial response pending independent confirmation. The same trial has expanded to investigate the efficacy and safety of two dosing regimens (240 mg 5 days on/9 days off vs 80 mg daily) of foretinib for PRCC. Of 37 enrolled patients in the 5-day-on/9-day-off cohort, 35 were evaluable; 4 patients experienced confirmed partial responses and 27 had stable disease. Enrollment is incomplete in cohort 2; however, among 9 evaluable patients, 2 had partial responses and 7 had stable disease. The investigators concluded that foretinib was well tolerated and displayed promising antitumor activity. Therefore, it appears that foretinib may be an effective therapy for PRCC. The final results from this study are eagerly awaited.
Sporadic papillary RCC
Pathology and Molecular Biology
Sporadic PRCC is itself a heterogeneous entity with at least 2 and possibly 3 distinct subtypes, both at the morphologic and genetic levels, which appear to have different clinical characteristics. As might be expected, most of these tumors have a papillary, tubular, or tubulopapillary growth pattern.
From a histologic standpoint, two different subtypes of PRCC are identified, type 1 with small cells and pale cytoplasm and type 2 with large cells and eosinophilic cytoplasm. Similarly, these two subtypes have distinct cytogenetic and molecular profiles that distinguish them from other renal epithelial tumors. Although only about 10% of sporadic type I PRCC have been reported to show somatic mutations in the c-MET gene, a genetic abnormality commonly seen as a germline mutation in hereditary cases, the c- Met pathway can be activated in many sporadic PRCC in the absence of c- Met mutation. The group from the National Institutes of Health described the genetic abnormality associated with the hereditary form of the type 2 papillary RCC, consisting of mutations in the fumarate hydratase ( FH ) gene. The contribution of this mutation to the pathogenesis of sporadic papillary type 2 RCC remains unknown.
More recently, Yang and colleagues proposed a refinement of the former (type I/type II) classification and introduced a molecular classification. Using gene expression profiling, they identified two highly distinct molecular PRCC subclasses with morphologic correlation. The first class, with excellent survival, corresponded to 3 histologic subtypes: type 1, low-grade type 2, and mixed type 1/low-grade type 2 tumors. The second class, with poor survival, corresponded to high-grade type 2 tumors. Dysregulation of G1-S and G2-M checkpoint genes were found in class 1 and 2 tumors, respectively. c-Met was differentially expressed, with higher expression in class 1 tumors. This refined classification of PRCC based on morphologic and molecular characteristics may be more relevant and is likely to aid diagnosis, prognosis, treatment, and analysis of clinical trials for advanced PRCC.
Treatment
Sunitinib inhibits the RTKs VEGFR2, platelet-derived growth factor receptor (PDGFR), FLT-3, and c-KIT ( Table 1 ). A dose of 50 mg orally once a day for 4 weeks followed by a 2-week break was the recommended phase 2 dose based on two phase 1 studies. It has subsequently been shown to significantly increase progression-free survival (PFS) in patients with metastatic CCRCC and has become a first-line standard of care for these patients.
| Agent | Target |
|---|---|
| Sorafenib | VEGFR2, VEGFR3, PDGFR, FLT-3, c-KIT, CRAF, wtBRAF, V600E BRAF |
| Sunitinib | VEGFR2, PDGFR, FLT-3, c-KIT |
| Temsirolimus | mTOR |
| Erlotinib | EGFR |
| Foretinib (GSK1363089) (previously XL880) | MET, VEGFR2 |
A worldwide expanded access trial of sunitinib has been undertaken, with a primary purpose to make the drug available to patients before regulatory approval. More than 4000 patients have been enrolled into this study, giving an important database especially for subgroup analysis. In May 2007, Gore and colleagues presented data on 2341 patients, the majority of whom (78%) had received prior cytokine therapy. A subgroup analysis of patients with non–clear-cell histology was performed and 276 patients (11.8%) with non–clear-cell histology were identified, although distinction between different subtypes was not made. A response rate of 5.4%, clinical benefit (defined as response and stable disease >3 months) of 47% and median PFS of 6.7 months was seen in this subgroup. This result compared with an overall response rate for the entire patient group of 9.3%, clinical benefit of 52.3%, and median PFS of 8.9 months. The investigators concluded that sunitinib was active in the non–clear-cell subgroup; however, these data need to be interpreted with caution because of the nonrandomization of patients in the expanded access trial and the lack of pathology verification.
In light of the results of the retrospective subgroup analysis, further trials have been initiated to provide additional data on sunitinib activity in NCCRCC. In 2008, Plimack and colleagues reported preliminary results from a phase 2 study of sunitinib in patients with NCCRCC. In a cohort of 26 patients of whom 13 had PRCC there were no objective responses, although 8 patients did experience stable disease. Moreover, the response rate and median PFS (48 days) were disappointing. Recently, updated results from this trial have been reported. The trial has been expanded to include 48 patients, with analysis focused on the patients with PRCC (23). Unfortunately, the results remained disappointing; among the PRCC patients the median PFS was 1.6 months (95% confidence interval [CI] 1.3–12), the median overall survival (OS) was 10.8 months (95% CI, 6.2 to not evaluable), and no major responses were observed, with the best response being stable disease (seen in 8 patients).
The SUPAP study is another phase 2 trial investigating sunitinib activity in type 1 and 2 PRCC. Twenty-eight patients were enrolled, and of the 23 patients with type 2 PRCC, 1 had a partial response and 13 had stable disease (lasting for ≥12 weeks in 4 patients). Five patients had type 1 PRCC, and although none experienced a partial response, 3 had stable disease. Based on these results, the investigators concluded that sunitinib did have some activity in PRCC, albeit inferior compared with CCRCC.
These conclusions have been supported by the results of another phase 2 study conducted in a cohort of 23 NCCRCC patients by Molina and colleagues. There were 8 patients with PRCC, and in this subgroup no partial responses were seen, with a median PFS of 5.6 months (95% CI 1.4–7.1). The data from recent phase 2 studies has therefore tempered the initial optimism raised by the retrospective subgroup analysis, and it appears that sunitinib at best has modest activity in PRCC. Nevertheless, there are still several ongoing phase 2 trials investigating sunitinib therapy for PRCC, and their results will be useful in clarifying the role of sunitinib in NCCRCC ( Clinicaltrials.gov identifier NCT00465179, NCT01034878, and NCT01219751). One study of 9 patients from Korea was preliminarily presented at the 2011 Genitourinary Cancers Symposium, and showed a response rate of 38% and a time to progression of 6.4 months. The investigators considered the primary end point has been met, and suggested that sunitinib has promising activity in patients with NCCRCC.
Sorafenib inhibits the RTKs VEGFR2, VEGFR3, FLT-3, c-KIT, and PDGFR, and the nonreceptor serine threonine kinases BRAF and CRAF (see Table 1 ). The BRAF and CRAF kinases are members of the RAF/MEK/ERK signaling cascade, which is involved in the survival and proliferation of tumor cells and is a therapeutic target in cancer, although it is not known to be of major importance in RCC. Sorafenib has subsequently been shown to significantly increase PFS in patients with metastatic CCRCC who had progressed on cytokine therapy, and is licensed for the treatment of metastatic RCC.
Ratain and colleagues were among the first to administer sorafenib for metastatic PRCC. In a phase 2 randomized discontinuation study; they treated 15 PRCC patients out of a total of 202 patients. From this subgroup, 2 patients achieved a partial response and 3 had tumor shrinkage of 25% to 49%; this was comparable to the entire population and indicated sorafenib activity in PRCC.
In one of the largest detailed series to date, Choueiri and colleagues reported on the efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe RCC. This retrospective analysis identified 53 patients who had been treated with either sunitinib or sorafenib at 5 different cancer centers in the United States and France. In contrast to the expanded access studies, expert genitourinary pathologists from each institution reviewed the cases to confirm the histopathological diagnosis of NCCRCC. Forty-one patients had PRCC; 13 were treated with sunitinib and of these, 2 patients achieved a partial response (15% response rate), with durations of 12 months and more than 8 months. No responses were seen in the 28 patients treated with sorafenib. In total, 27 patients (68%) achieved stable disease for more than 3 months after 2 cycles of treatment with sunitinib or sorafenib. Minor responses ranging from −4% to −25% were seen in 9 patients. PRCC patients had a PFS of 7.6 months, and it was observed that treatment with sunitinib resulted in a superior PFS compared with sorafenib (PFS 11.9 vs 5.1 months, respectively; P <.001), and this remained statistically significant even after adjusting for other important prognostic factors in metastatic RCC such as hemoglobin and the number of metastatic sites.
A worldwide expanded access trial of sorafenib has also been undertaken. Response data on the Advanced Renal Cell Carcinoma Sorafenib (ARCCS) expanded access trial in North America has recently been reported on 1891 patients out of a total of 2504 patients enrolled. This study contained a subgroup of 107 PRCC patients with valid data. Within this subgroup, 3 patients (3%) exhibited partial responses, with 87 patients (81%) experiencing stable disease lasting for at least 8 weeks. This study also included an extension protocol for which NCCRCC patients and patients who had not received prior therapy were eligible, although specific distinctions between NCCRCC subtypes were not made. Data were available for 248 patients in this extension protocol; NCCRCC patients (n = 26) had a PFS of 46 weeks (95% CI 30–59; censorship rate 39%) compared with first-line patients who had a PFS of 36 weeks (95% CI 33–45; censorship rate 56%). Overall in the whole trial, toxicities for NCCRCC patients did not differ from those seen in patients with CCRCC, and sorafenib was well tolerated in both groups. Moreover, it was concluded that sorafenib appeared to have activity against PRCC.
A similar European expanded access study of sorafenib was undertaken (the European ARCCS). This study included 118 patients with PRCC of whom 104 were evaluable for response. The disease control rate was 66.4% and the median PFS was 5.8 months for PRCC compared with 75.7% and 7.5 months for patients with CCRCC, respectively.
Overall, currently available data from retrospective and expanded access studies suggest that sorafenib may possess activity against PRCC. Smaller-scale studies have also supported this impression. Unnithan and colleagues investigated cell lines established from primary and metastatic tumors from a patient with type II PRCC, and reported that sorafenib inhibited cell growth and expression of angiogenic genes such as VEGF and PDGF. Given its apparent promising activity, further trials may be necessary to confirm whether sorafenib is suitable for NCCRCC therapy.
Temsirolimus, a derivative of sirolimus (rapamycin), inhibits mTOR (see Table 1 ). Temsirolimus has been studied in a 3-arm phase 3 study comparing temsirolimus, interferon-α (IFN-α), and the combination of the two agents as first-line therapy for poor-risk patients with metastatic RCC. Response rates were similar in all 3 arms and ranged between 7% and 11%, but median OS was longer in the temsirolimus single-agent arm in comparison with the other 2 arms (10.9 months for temsirolimus, 7.3 months for IFN-α, and 8.4 months for the combination; hazard ratio [HR] 0.73, P = .0069 for single-agent temsirolimus). The investigators concluded that temsirolimus as a single agent significantly improves OS of patients with metastatic RCC and poor-risk features as compared with IFN-α, but the combination of the two drugs does not improve OS.
In this study, approximately 20% of all patients had non–clear-cell histology. Of these patients, 75% had PRCC. A subset analysis has been performed to determine the effect of temsirolimus versus IFN-α on OS and PFS in patients with clear-cell or other histologies. For NCCRCC patients (n = 73), those in the temsirolimus group had a longer OS and PFS than those in the IFN-α group (median OS 11.6 vs 4.3 months, respectively; HR 0.49; median PFS 7.0 vs 1.8 months, respectively; HR 0.38). Thus, it seems that temsirolimus may benefit patients irrespective of histology and warrants further study in patients with non–clear-cell histologies. Unfortunately, this study had no central review of the histology and therefore there was no detailed differentiation between different non–clear-cell subtypes.
More recently, Yang and colleagues performed further retrospective analysis, focusing on quality of life data gathered using the EuroQoL-5D utility score (EQ-5D index) and EQ-5D visual analog scale (EQ-VAS). It was observed that the mean EQ-5D score was higher in the temsirolimus arm compared with the IFN-α arm in NCCRCC patients.
The possibility that mTOR inhibitors have clinical activity regardless of RCC histology has led to the development of studies aimed at patients with non–clear-cell histology, and a phase 2 trial comparing temsirolimus against sunitinib as first-line therapies is currently recruiting (NCT00979966). Everolimus is another mTOR inhibitor that is being investigated by several trials. Most notably, the RAPTOR study aims to evaluate everolimus as a first-line therapy for PRCC (NCT00688753). Other ongoing trials are also investigating the use of everolimus alone, or in comparison with sunitinib, for treatment of NCCRCC (NCT00830895, NCT01185366, NCT01108445). The randomized phase 2 studies comparing mTOR inhibitors with sunitinib may help to clarify the relative role of each agent in NCCRCC.
The rationale for the use of erlotinib, an oral EGFR TKI, in PRCC stems from a study by Perera and colleagues. These investigators demonstrated that blockade of the EGFR by an anti-EGFR monoclonal antibody resulted in significant growth inhibition in NCCRCC-derived cell lines, suggesting that EGFR blockade may provide a potential therapeutic approach. In a study led by the Southwest Oncology Group (SWOG), Gordon and colleagues treated 45 patients with PRCC with erlotinib (150 mg/d). Five patients achieved a partial response for an overall response rate of 11% (95% CI 3–24) with a disease control rate (DCR) of 64% (5 partial response + 24 stable). Median OS time was 27 months (95% CI 13–36). There was no correlation between EGFR expression and disease outcome, and the drug was generally well tolerated. Although the RECIST response rate of 11% did not exceed prespecified estimates (≥20% response rate) for further study, single-agent erlotinib yielded encouraging DCR and OS results. As a result of its promising activity, two phase 2 trials are now under way to investigate erlotinib alone and in combination with bevacizumab in patients with PRCC (NCT01130519, NCT00060307).
Foretinib (GSK1363089) is a novel inhibitor of RTKs targeting MET and VEGFR. In a phase 1 study partial responses were noted in 2 out of 4 patients with PRCC, lasting for longer than 48 and 12 months. This finding has led to the initiation of a multicenter phase 2 study of foretinib (240 mg/d orally for 5 days on/9 days off) in patients with histologically confirmed PRCC. After enrollment, patients were stratified into two strata based on the presence or absence of a genetic aberration in c-MET (A: evidence of c-MET pathway activation; B: without evidence of activation). Thirty-one patients were enrolled (15 strata A and 16 strata B), and of 25 evaluable patients, 24 had at least stable disease and 20 had decreases in tumor size (range 4%–35%). Two patients had confirmed partial response and 2 had unconfirmed partial response pending independent confirmation. The same trial has expanded to investigate the efficacy and safety of two dosing regimens (240 mg 5 days on/9 days off vs 80 mg daily) of foretinib for PRCC. Of 37 enrolled patients in the 5-day-on/9-day-off cohort, 35 were evaluable; 4 patients experienced confirmed partial responses and 27 had stable disease. Enrollment is incomplete in cohort 2; however, among 9 evaluable patients, 2 had partial responses and 7 had stable disease. The investigators concluded that foretinib was well tolerated and displayed promising antitumor activity. Therefore, it appears that foretinib may be an effective therapy for PRCC. The final results from this study are eagerly awaited.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree