Fig. 10.1
The main clinical characteristics of the patients with TH action defects, RTHα or RTHß. ADHD attention-deficit, hyperactivity disorder
10.2.1.1 Goiter
Diffuse or multinodular goiter is a common finding in RTH β, independently from the presence of clinical symptoms. An increased biological activity of circulating TSH molecules may favor the formation of goiter in RTH β subjects with normal TSH levels [26]. In RTH β patients treated by surgical ablation, the goiter commonly relapses with nodular alterations and gross asymmetries, requiring additional surgery or radioiodine.
10.2.1.2 Cardiovascular Symptoms
Approximately 75 % of RTH β patients exhibit palpitations and tachycardia at rest. Predominance of TRα may explain the presence of partially hyperthyroid response in the heart, as the dominant negative effect exerted by mutant TRβs on the normal receptors should be weaker than in other tissues. The finding that some indices of cardiac systolic and diastolic function (e.g., heart rate, stroke volume, cardiac output, diastolic filling, maximal aortic flow velocity) showed values that are intermediate between normal and hyperthyroid subjects supports this hypothesis. However, the normal values of other parameters (e.g., ejection and shortening fractions of the left ventricle, systolic diameter, and left ventricle wall thickness) suggest an incomplete response of the heart to the high TH concentrations. In addition, systemic vascular resistance and arterial stiffness are increased in RTH β, as seen in subclinical hypothyroidism, thus indicating a more complex derangement of cardiovascular function. A reduced insulin sensitivity and dyslipidemia have been documented in a number of patients, suggesting an increased cardiovascular risk in RTH β [27–30].
10.2.1.3 Skeletal Abnormalities
Similarly to the cardiovascular system, also the bone is affected by a mix of hypothyroid and thyrotoxic manifestations in RTH β. Studies performed in animal models suggest that skeletal thyrotoxicosis, due to elevated circulating thyroid hormone levels which overstimulate the intact TRα1 signaling pathway, may be responsible for bone abnormalities in RTH β [31].
In humans, dysmorphic skeletal features, such as “stippled epiphyses,” dysmorphic facies, and winged scapulae, have been documented only in the cases harboring complete TRβ resistance due to homozygous deletion of TRβ gene.
Delayed bone maturation and growth are present in about one third of children with RTH β; however, the final adult height seems unaffected.
A decreased bone mineral density and increased risk of fractures have been reported in adult RTH β. Conversely, the normal levels of the markers of bone turnover may imply a reduced bone formation rate resulting in a low peak bone mass similar to that observed in childhood hypothyroidism.
10.2.1.4 Metabolism
Low body mass index (BMI) is reported in about 30 % of RTH β children, in spite of the hyperphagia and the enhanced energy intake.
Basal metabolic rate (BMR) has been found normal or even increased. Indirect calorimetry assessment showed enhanced resting energy expenditure (REE), either in adults or children with TRβ mutations. This increase was intermediate between euthyroid and thyrotoxic subjects. Skeletal muscle and myocardium, in which the TRα isoform expression is prevalent, seem responsible for increased energy expenditure, as suggested by the correlation between mean heart rate and REE in both RTH and thyrotoxicosis. In both these conditions, TH excess was associated with uncoupling between tricarboxylic acid cycle activity and ATP synthesis in vivo, as measured by magnetic resonance spectroscopy [30].
10.2.1.5 Immune System
An increased frequency of respiratory infections (pulmonitis and infections of the upper respiratory tract) has been reported in RTH β patients, compared to their unaffected relatives. This susceptibility has been related to reduced immunoglobulin concentrations but may also derive from an abnormal regulation of granulocytes and lymphocytes that express TH receptors.
10.2.1.6 Neurological System
It has been hypothesized that in RTH β an uncompensated hypothyroidism at an early stage may be responsible for defects of neuroanatomical development.
Few data are available about the brain anatomical abnormalities associated with RTH β. A single MRI study in 43 RTH β patients found, in male patients, an increased frequency of cerebral anomalies of the left hemisphere, particularly an extra or missing gyrus in the parietal bank of the Sylvian fissure or multiple Heschl’s transverse gyri in the primary auditory cortex when compared to unaffected relatives. No patent abnormalities were found in female patients [32].
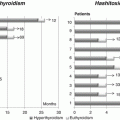
Although severe mental retardation (IQ <60) is uncommon (only 3 %), about 30 % of affected subjects display a mild learning disability (IQ <85). In particular, either the verbal or the performance component were impaired compared with controls [33]. Some authors have reported in their RTH β cohort a high frequency of attention deficit hyperactivity disorder (ADHD). This finding has not been confirmed by other groups, but it is possible that the low IQ may be responsible for ADHD manifestations, more than RTH β per se. In addition, an increased frequency of delayed developmental milestones and language disorders has been found in RTH β patients, compared to their unaffected relatives [33–36]. The association with Tourette syndrome has been also described [63] (Fig. 10.1).
The neuroanatomical regions involved in attention and vigilance are located in the right lateral prefrontal cortex, in the parietal lobe, and in anterior cingulate. Consistently, Matochik et al. found a severe impairment on an attention auditory discrimination task in adults with RTH β compared to controls. The PET scan performed during this task demonstrated the presence of an increased metabolic activation of the anterior cingulate in RTH β. The reduction of the functional activity in this brain area and the subsequent activation of other structures, such as the frontal cortex, are required for an efficient performance on complex attention tasks. However, it is not clear whether these functional anomalies are related to a defect in brain development or may be a consequence of the elevated levels of thyroid hormones via overstimulation of the TR-α [37].
Patients with homozygous deletion of THRB display a phenotype characterized by deaf-mutism due to sensorineural hearing loss, delayed bone maturation, stippled epiphyses, goiter, and high levels of circulating thyroid hormone in the presence of a normal TSH [2, 22].
Interestingly, these patients with deletions do not display growth delay, mental retardation, or cognitive impairment, while the five cases, homozygous for missense mutations of THRB, are invariably associated with a mild to severe intellectual impairment, neuropsychomotor retardation, goiter, hyperactivity, tachycardia, and hearing loss as the extreme manifestations of resistance [38–40].
“Conventional” heterozygous mutations, resulting in a premature stop codon with the consequent production of a TR-β lacking a number of residues in the C-terminal, also display a strong dominant-negative effect in vitro and are often associated with a severe clinical phenotype, including mental retardation [41–43].
10.2.1.7 Visual System
In animal models, the deletion of the TRβ2 isoform produces a selective loss of M cone photoreceptors resulting in abnormal color vision. In particular, during embryogenesis, TR-β seems responsible for the photoreceptor distribution in the retina, inhibiting the S-opsin and committing the differentiation of M-opsin photoreceptor. However, no abnormalities of color sensitiveness have been identified in “conventional” RTH β patients with heterozygous TRβ mutations. Patients with homozygous deletion of THRB gene [2, 22] are color blind, while in one patient with compound heterozygous mutation (R338W in exon 9 and R429W in exon 10 of THRB gene), an abnormal electroretinographic pattern was found, characterized by a normal scotopic response and a reduced photopic response. In particular, this patient showed a small-amplitude b-wave to a red flash and a larger-amplitude b-wave to the blue flash, similar to what is commonly described in the enhanced S cone syndrome [44].
10.2.1.8 Hearing System
An increased incidence of conductive or sensorineural hearing impairment, which may contribute to the defective speech development, has been reported in some RTH β children. The pathophysiology of these abnormalities is composite, being the conductive defect due to the higher susceptibility to upper airway infection of RTH β children, whereas the defective TRβ expression may be responsible for the cochlear dysfunction [45].
Noteworthy, mice with targeted disruption of the TRβ locus develop profound sensorineural hearing loss, thus suggesting an important role of TH in the development of the hearing system.
10.2.1.9 Other Features
In mothers affected with RTH β, there is a higher rate of miscarriage and intrauterine growth retardation of unaffected offspring, thus suggesting that intrauterine exposure to high TH levels does have adverse effects on the fetus.
There is only one patient, homozygous for TRβ mutation, in whom RTH β may have contributed to death: this patient had resting pulse of 190 beats/min and died from cardiogenic shock complicated by septicemia.
Coexistence of TSH-secreting pituitary adenomas (TSHomas) and RTH β has been suggested in only two cases. The impaired TH feedback in the pituitary may lead to a continuous stimulus to thyrotropes to synthesize and secrete TSH molecules, which may play a role in the development of pituitary tumors. However, the pituitary lesions associated to RTH β appear to be pituitary “incidentalomas” [46]. Interestingly, somatic mutations of TR-beta have been found in two TSH-secreting pituitary adenomas [47, 48] but never on the germinal DNA of patients with TSHomas.
Occasionally, RTH β occurs in association with autoimmune thyroid disorders, such as Graves’ disease or Hashimoto’s thyroiditis. The occurrence of anti-TPO or anti-TSH receptor autoantibodies in RTH subjects has been described. Recent data suggest that the individuals with RTH β due to TRβ gene mutations have an increased likelihood of AITD compared to unaffected relatives [49]. The reason for this association seems related with the hyperstimulation, via TR-alpha, of the cells of the immune system.
The RTH patients, who develop Graves’ disease, undergo a progressive increase in goiter size along with frank symptoms of thyrotoxicosis. The further elevation of TH levels causes TSH secretion to be totally inhibited. Conversely, hypothyroidism may occur in the presence of normal serum TH concentrations, as a consequence of Hashimoto’s thyroiditis.
10.2.2 Differential Diagnosis of RTH β
RTH β shares the same biochemical features of patients with TSH-omas, and occasionally with Familial Dysalbuminemic Hyperthyrotoxinemia (FDH) (Table 10.1). Since these two diseases have completely different therapeutic and management approaches, their differential diagnosis is mandatory [46]. The presence of the same abnormal biochemical pattern of thyroid function in other first-degree relatives supports the diagnosis of RTH β, since familiar cases of TSHoma have never been reported (except for four families in a setting of multiple endocrine neoplasia 1). In these cases, molecular analysis of the THRB gene makes a definitive diagnosis in 85–90 % of cases of RTH β.
Table 10.1
Genetic disorders characterized by increased serum thyroid hormones levels and detectable TSH concentrations
GENE | Free T4 | Free T3 | TSH | Total reverse T3 | SHBG | |
|---|---|---|---|---|---|---|
Familial dysalbuminemic hyperthyroxinemia (FDH) | ALB | Na | Na | N | ↑ | N |
Resistance to thyroid hormone (RTH β) | THRB | ↑ | ↑ | N or slightly ↑ | ↑ | N |
Defect of THRA gene (RTH α) | THRA | borderline or slightly↓ | ↑ | N | ↓ | ↑ |
Defect of thyroid hormones transport (Allan-Herndon-Dudley syndrome) | MCT8 | slightly ↓ | ↑ | N or slightly ↑ | ↓ | ↑ |
Defect of thyroid hormones metabolism (SBP2 deficiency) | SBP2 | ↑ | N or slightly ↓ | N or slightly ↑ | ↑ | N |
Although different clinical parameters have been proposed (basal metabolic rate, systolic time intervals, Achilles reflex time) in order to discriminate among these two conditions, the clinical presentation of patients with RTH β may be similar to those with TSHoma [46], though the onset of central hyperthyroidism generally occurs beyond 30 years of age in the latter condition.
In patients with TSH-omas, serum levels of glycoprotein hormone α-subunit (α-GSU) and α-GSU/TSH molar ratio are elevated, whereas in RTH β patients both indices are in the normal range.
To assess the degree of resistance in specific target tissues, different in vitro parameters have been proposed. Particularly, SHBG and ICTP are in the hyperthyroid range in patients with TSH-oma and within the normal range in RTH β. The sensitivity and specificity of these tests is improved, when assessed after T3 suppression test, performed with oral administration of supraphysiological doses of T3 (50 μg/day for 3 days, followed by 100 μg/day for another 3 days and then 200 μg/day for another 3 days) [7]. In RTH β patients, the increase of peripheral markers of TH actions and heart rate is blunted in comparison to normal subjects, thus definitively confirming the presence of resistance to TH action.
The TRH test (IV injection of TRH 200 μg) has been also widely used: in the majority of patients affected with TSH-oma, TSH and α-GSU levels do not increase after TRH injection, whereas RTH β subjects show normal response of TSH.
T3 inhibitory test, performed as reported above or administering T3 for 8–10 days at the dose of 80–100 μg/day, may show a full inhibition of TSH levels in RTH β patients but persistent TSH response to TRH, carried out at the end of T3 administration. Since none of these tests have a clear diagnostic cutoff value, the combination of them, if possible, increases the specificity and sensitivity of the diagnostic process.
The administration of long-acting somatostatin analogues (e.g., long-acting Octreotide-LAR 30 mg intramuscularly every 28 days) for at least 2 months can be useful in the differential diagnosis in problematic cases of central hyperthyroidism. Chronic administration of long-acting somatostatin analogues in patients with central hyperthyroidism caused a marked decrease of FT3 and FT4 levels in patients with TSH-oma (>30 % of pretreatment values), while patients with PRTH did not respond at all.
Pituitary MRI is required in case of not univocal results with other tests; however, the detection of pituitary lesions does not definitely rule out the diagnosis of RTH β. In fact, pituitary lesions are quite a common finding (20–25 % of MRI performed for other reasons) in the general population. These lesions are usually considered as “pituitary incidentalomas,” especially when a hypothalamic-pituitary dysfunction has been excluded. The presence of a microadenoma in combination with lack of TSH response to dynamic tests and high levels of α-GSU or α-GSU/TSH molar ratio strongly sustains the diagnosis of a TSH-oma.
10.2.3 Therapy
There is currently no definite therapy to correct the molecular defect causing RTH β, and in most patients a specific treatment is not even necessary, as goiter may be the only sign of the disease. The high levels of circulating free TH may be able to compensate for the resistance in several of the peripheral tissues but may create a thyrotoxic state in several others.
Patients with tachycardia and palpitations at rest may benefit by the use of cardioselective β-blockers (atenolol or others). In the event of severe thyrotoxic symptoms, not responding to β-blockers, a reduction of thyroid hormone levels may be beneficial. This can’t be achieved using antithyroid drugs, because the consequent increase of TSH levels may determine goiter enlargement. The treatment of choice in such cases is the administration of thyromimetic compounds, such as 3,5,3′-triiodothyroacetic acid (TRIAC), which through the feedback mechanism reduces TSH secretion and causes a slight decrease of circulating T4 levels (values of T3 are unreliable as TRIAC cross-reacts in T3 measurement methods). As a consequence of its weaker effects on peripheral tissues, TRIAC reduces the thyrotoxic signs and symptoms, particularly at the heart level. TRIAC has been shown to be beneficial in both children and adult patients with RTH β at the dose of 1.4–2.8 mg/day, fractionated in two or three administrations [50].
The use of dopaminergic drugs and somatostatin analogues has limited success because TSH secretion rapidly escapes the inhibitory effects of both drugs, as the T4 reduction triggers the much more potent stimulatory effect of TH negative feedback mechanism.
Although controversial, in children with signs of growth or mental retardation, the administration of supraphysiological doses of L-T4 to overcome the high degree of resistance present in some tissues can be beneficial. Supraphysiological doses of thyroid hormones are also necessary in patients treated with total thyroidectomy for a missed diagnosis of RTH β. The use of high doses of L-T4 requires a careful monitoring of patients, assessing the indices of peripheral thyroid hormone action.
Recently, TRβ selective agonists (GC1, eprotirome) have been developed and could be beneficial for some abnormalities (dyslipidemia) found in RTH β. Unfortunately, the development program on this drug has been discontinued after the evidence of cartilage damage after 12 months administration in dogs. In addition, there is evidence that eprotirome may induce liver injury in humans [51].
10.3 Resistance to Thyroid Hormones due to THRA Mutations
10.3.1 General Features of RTH α
Recently, the first three families with TH resistance due to TR-alpha (RTH α) have been described [5, 52–55]. Similar to that described in animal models [56, 31], these subjects present variable features of hypothyroidism associated with normal TSH levels (Fig. 10.1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree