Fig. 5.1
Model for explaining the influence of cancer and its treatments on common behavioral alterations including fatigue and cognitive dysfunction [Reproduced with permission from Miller et al. (2008)]
These behavioral changes have been collectively described as “sickness behavior” and are thought to represent a motivational shift designed to facilitate recovery and prevent the spread of infection (Dantzer and Kelley 2007; Irwin and Cole 2011). In humans, pharmacologic doses of cytokines given for treatment of cancer or hepatitis C are associated with significant increases in fatigue, cognitive problems, and other markers of sickness (depressed mood, sleep disturbance) (Capuron et al. 2000; Kirkwood 2002; Valentine et al. 1998). Experimental studies of cytokine induction in healthy individuals have documented similar effects, with subjects reporting increased fatigue and cognitive disturbance following endotoxin administration that are correlated with elevations in circulating concentrations of pro-inflammatory cytokines (Reichenberg et al. 2001; Spath-Schwalbe et al. 1998). Further, pharmacologic agents that block the pro-inflammatory cytokine TNFα lead to reduced fatigue among individuals with inflammatory conditions (Tyring et al. 2006), and in pilot studies with cancer patients (Monk et al. 2006) (though fatigue can also be a side effect of these agents in certain patient populations). Together, this evidence provides a strong biological rationale for inflammation as a potential mechanism underlying cancer-related fatigue and cognitive disturbance.
Studies of Inflammation and Fatigue in Cancer Patients
In the cancer context, investigators have proposed that tumors and the treatments used to eradicate them can activate the pro-inflammatory cytokine network, leading to symptoms of fatigue and cognitive disturbance (Cleeland et al. 2003; Miller et al. 2008; Seruga et al. 2008). In the pre-treatment period, the tumor itself may be a source for pro-inflammatory cytokines (Aggarwal et al. 2009; Coussens and Werb 2002) while during treatment, cytokines may be produced in response to tissue damage from surgery, radiation, or chemotherapy (Aggarwal et al. 2009; Stone et al. 2003). The inflammatory response may persist well after treatment completion as the host tries to deal with persisting pathogenesis and alterations in homeostasis.
A growing number of studies have examined the association between circulating markers of inflammation and fatigue during and after breast cancer treatment. In a study of breast cancer patients assessed prior to chemotherapy (but after surgery), fatigue was associated with elevations in CRP, a marker of systemic inflammation (Pertl et al. 2013). In a study of breast and prostate cancer patients undergoing radiation therapy, we found that patients reported increases in fatigue that were correlated with increases in circulating inflammatory markers (CRP, IL-1 receptor antagonist) (Bower et al. 2009). Similarly, increases in fatigue were correlated with increases in the inflammatory cytokine IL-6 among breast cancer patients undergoing chemotherapy (Liu et al. 2012). Documenting an association between inflammatory markers and on-treatment-related fatigue is complicated by dynamic changes in the cellular immune system and inflammation that occur during the acute phase of cancer treatment. Investigators have found more reliable associations between inflammatory activity and fatigue after treatment completion. In a series of cross-sectional studies with breast cancer survivors, we have documented elevations in inflammatory markers among women who report elevated fatigue at 1 month (Bower et al. 2011b), 2 years (Collado-Hidalgo et al. 2006), and 5 years (Bower et al. 2002) post-treatment. Consistent with these results, several other groups have found significant elevations in CRP among breast cancer survivors with persistent fatigue (Alexander et al. 2009; Alfano et al. 2012; Orre et al. 2011). At the molecular level, leukocytes from fatigued breast cancer survivors show increased expression of genes encoding proinflammatory cytokines and other mediators of immunologic activation, as well increased activity of proinflammatory NF-κB/Rel transcription factors, which might structure the observed differences in the expression of inflammation-related genes (Bower et al. 2011a).
Studies of Inflammation and Cognitive Function in Breast Cancer Patients
In parallel to the studies of fatigue, there are increasing reports that have focused on the potential role of inflammation in the etiology of cognitive impairment after breast cancer. Early reviews of potential mechanisms identified inflammation as a possible etiology (Ahles and Saykin 2007) and studies in rodents provide strong support for inflammatory mechanisms (Seigers and Fardell 2011). While some chemotherapeutic agents may cross the blood brain barrier and cause direct toxicity (e.g., especially the CMF regimen, with methotrexate and fluorouracil), the mechanism by which both chemotherapy and radiation cause injury is likely through the production of reactive oxygen species and tissue damage, that result in systemic inflammation as well as stimulation of local microglial inflammation within the brain. Indeed, several studies of breast cancer patients have demonstrated relationships between systemic levels of inflammation and brain imaging structural and metabolic changes (Kesler et al. 2013b; Pomykala et al. 2013). Animal models studies support these findings (Seigers et al. 2013), and an inflammatory basis of cognitive changes associated with cancer treatments would be consistent with age related cognitive changes of which this may be a manifestation (Ahles 2012). Since only a subgroup of patients with breast cancer appear to be vulnerable to cognitive difficulties, as with age-related variation in cognitive decline, similar host factors and susceptibilities may be relevant (see below).
To develop an understanding of the potential role of inflammation and cognitive dysfunction in women with breast cancer, we recruited a cohort of women with newly diagnosed breast cancer who had completed primary adjuvant chemotherapy and/or radiation therapy, but enrolled prior to the start of endocrine therapy if planned. The Mind Body Study (MBS) cohort of 191 patients was less than 66 years of age, and excluded women with significant depressive symptoms, history of central nervous system disorders, conditions with chronic inflammation, or with use of immunosuppressive therapy (see details in Bower et al. 2011b; Ganz et al. 2013a, b). We observed post-treatment elevations of soluble TNFα receptor II (sTNFR2) levels at study enrollment that declined over the subsequent 12 months of follow-up, with elevations only noted in the patients who had received chemotherapy (Ganz et al. 2013a). We should note that there was a parallel association between fatigue and sTNFR2 in this same sample at the baseline assessment (Bower et al. 2011b), and we see the co-occurrence of these two symptoms in the longitudinal follow-up of this sample (unpublished data). The changes in TNF over the 12 months were correlated with self-reported memory complaints, as well as changes in PET scan glucose metabolism in a small subgroup of patients, with normalization of metabolism in the inferior frontal gyrus as TNF levels decreased between baseline and 12 months later. More detailed evaluation of sTNFR2 and other proinflammatory cytokines in the PET scan study are reported separately in an additional publication, where we observed positive correlations between metabolism in the medial prefrontal cortex and anterior temporal cortex with both memory complaints and cytokine markers only in patients who received chemotherapy (Pomykala et al. 2013). Of note, Kesler et al. (2013b) have found an association between decreased hippocampal volume on MRI in breast cancer survivors and elevated TNFα and IL-6, along with decreased verbal memory performance on cognitive testing, in comparison to a healthy control group.
Host Factors that May Increase Risk for Fatigue
Although cancer-related fatigue is common, it does not affect all patients (see Table 5.1). Clinicians have no doubt observed that certain patients are more susceptible to fatigue, and empirical studies have now documented considerable variability in reports of fatigue before, during, and after treatment. This variability was nicely illustrated in a longitudinal prospective study of breast cancer patients who were followed for 6 months after cancer treatment (Donovan et al. 2007). Using growth mixture modeling, two groups of patients were identified on the basis of their fatigue scores. One group, which comprised approximately 30 % of the sample, reported consistently low levels of fatigue across the assessment period, including in the immediate aftermath of treatment. The other group reported elevated fatigue at treatment completion, which declined over the assessment period but remained significantly higher than the low fatigue group. Of note, disease- and treatment-related factors did not determine group membership in this study; instead, body mass index and coping strategies were significant predictors of group membership. Other studies have similarly found no evidence that cancer-related fatigue is associated with type of cancer treatment, particularly in the post-treatment period. Together, these findings strongly suggest that host factors play an important role in the development and persistence of cancer-related fatigue.
Table 5.1
Host factors associated with fatigue and cognitive dysfunction
Fatigue |
Pre-treatment fatigue |
Pre-treatment sleep disturbance |
History of depression |
Loneliness |
Early life stress |
Physical inactivity |
High body mass index |
Catastrophizing coping style |
Genetic factors (e.g., SNPs in inflammation-related genes) |
Neuroendocrine dysregulation |
Cognitive dysfunction |
Pre-treatment diminished cognitive reserve, low educational status |
History of head trauma |
Comorbid conditions (e.g., diabetes, vascular disease) |
Genetic factors (e.g., APOE-4, COMT, SNPs in inflammation-related genes) |
Older age (?) |
Longitudinal studies have begun to identify predictors of cancer-related fatigue. These include pre-treatment fatigue, pre-treatment sleep disturbance, history of depression, loneliness, early life stress, physical inactivity, and body mass index (Bower 2014). In addition, patients who engage in negative thoughts or “catastrophize” about their fatigue (e.g., I tell myself I don’t think I can bear the fatigue any more), report elevated fatigue during and after treatment. Thus, psychosocial and behavioral factors may set the stage for more severe and persistent cancer-related fatigue. Importantly, some of these factors are amenable to intervention, including physical inactivity, high BMI, and catastrophizing.
Genetic factors have also been linked to cancer-related fatigue. Most of the studies in this area have taken a candidate gene approach, focusing on single nucleotide polymorphisms (SNPs) in inflammation-related genes including IL1B, IL6, and TNF given evidence linking circulating inflammatory markers and fatigue. We examined whether polymorphisms in these genes were associated with fatigue within 1 month after treatment, using data from breast cancer survivors enrolled in the MBS study. Consistent with hypotheses, we found that women with the “high expression” versions of these genes reported higher levels of fatigue (Bower et al. 2013a). Similarly, in a small sample of breast cancer survivors assessed several years after treatment, polymorphisms in ILB and IL6 were associated with persistent post-treatment fatigue (Collado-Hidalgo et al. 2008). There is also preliminary evidence that polymorphisms in inflammation-related genes are associated with fatigue among patients undergoing radiation therapy, including many breast cancer patients (Aouizerat et al. 2009; Miaskowski et al. 2010).
Alterations in the HPA axis may contribute to cancer-related fatigue, either directly or through effects on inflammatory processes. We found that breast cancer survivors with persistent fatigue had a flatter diurnal cortisol slope (with elevated levels of cortisol in the evening) as well as blunted cortisol responses to psychosocial stress that were correlated with alterations in inflammatory activity (Bower et al. 2005a, b, 2007) Further, genome-wide transcriptional profiling of leukocytes from fatigued breast cancer survivors showed a marked down-regulation of genes with response elements for the glucocorticoid receptor, suggesting a state of functional GR resistance which may contribute the tonic upregulation of NF-κB observed in fatigued survivors (Bower et al. 2011a). Fatigue is also associated with alterations in the autonomic nervous system in breast cancer survivors, including lower heart rate variability (an indicator of parasympathetic activity) and elevated norepinephrine (an indicator of sympathetic activity) (Crosswell et al. 2014; Fagundes et al. 2011). Importantly, because all of these studies have been cross-sectional investigations of breast cancer survivors, it is impossible to determine whether neuroendocrine alterations play a causal role in the development and persistence of this symptom, or arise as a consequence of fatigue and inflammatory activity.
Longitudinal studies that examine risk factors for cancer-related fatigue are still quite limited and few have followed patients from pre-treatment in to the post-treatment period; fewer still have examined mechanisms that underlie effects of these risk factors on fatigue. To advance research in this area, longitudinal studies are required that track patients before, during, and after treatment and include comprehensive assessment of biobehavioral risk factors and underlying mechanisms. This approach will facilitate the identification of distinct trajectories of fatigue, risk factors for fatigue onset and persistence, and the mechanisms that underlie their effects, paving the way for targeted interventions.
Host Factors and the Risk for Cognitive Impairment
Less is known about the host factors associated with the risk of cognitive impairment after breast cancer treatments (see Table 5.1). Ahles and Saykin (2007) reviewed potential mechanisms for the development of cognitive changes and these included genetic susceptibility, endocrine factors (reductions in estrogen and testosterone), DNA damage and telomere length, cytokine dysregulation and disruption in the blood brain barrier. Among these mechanisms, genetic susceptibility has been studied by several groups. Ahles has reported on the association of the APOE-4 allele, found in Alzheimer’s disease, with cancer-related cognitive dysfunction in long-term breast and lymphoma survivors treated with chemotherapy (Ahles et al. 2003). In another sample of breast cancer patients followed prospectively, Small et al. (2011) found that patients with the catechol-o-methyltransferase (COMT) genotype Val + allele had greater cognitive difficulties with attention, verbal fluency and motor speed, with an interaction with chemotherapy for attention. COMT-Val + carriers are thought to metabolize dopamine more rapidly and this might be the putative mechanism. In our MBS study, we have found that a genetic risk score of SNPs for IL1B, IL6, and TNF was significantly associated with memory complaints as well as fatigue (Bower et al. 2013b). Other groups have also found similar associations (Merriman et al. 2013, 2014).
Other contributing factors could be those influences associated with age-related cognitive decline and cognitive reserve may be reduced in individuals with lower education or prior comorbid conditions leading to subclinical brain injury (Ahles 2012; Mandelblatt et al. 2014) (see Fig. 5.2). It is likely that the cognitive complaints that patients report after treatment exposure are a manifestation of having to work harder (recruit more areas of the brain) to retrieve information, multi-task, and perform executive tasks. These are similar to what happens with age-related cognitive decline (Maillet and Rajah 2013). In addition to these factors, age-related vascular disease, diabetes, and hormonal changes may contribute to these problems. However, it is most interesting the manifestations of symptomatic cognitive difficulties are most notable in younger women, similar to what is seen with fatigue. It may be that the everyday demands put upon younger women exacerbate these complaints, whereas older women may be less likely to notice subtle changes in function.
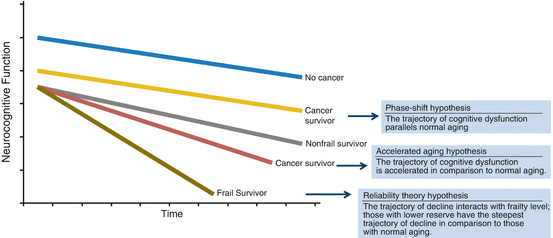

Fig. 5.2
Trajectories of cognitive decline based on theories of aging and frailty phenotype [Adapted from Mandelblatt et al. (2014)]
What Are the Potential Intervention Strategies to Consider for Management of Fatigue or Cognitive Complaints?
Interventions for Cancer-Related Fatigue
A diverse range of treatment approaches have been used to address cancer-related fatigue during and after cancer treatment, including physical activity, psychosocial, mind-body, and pharmacological interventions. Perhaps because the etiology of cancer-related fatigue is multi-factorial and still poorly understood, there is currently no “gold standard” for treatment of this symptom. Still, a number of these approaches have been shown to be beneficial in reducing cancer-related fatigue, as reviewed below. The recently published ASCO Guideline on Fatigue in Cancer Survivors outlines the intervention strategies that should be considered (Bower et al. 2014). A number of randomized controlled trials have examined the effect of exercise on cancer-related fatigue. Overall, meta-analyses of these trials indicate that exercise is effective in reducing fatigue, with effect size estimates ranging from −0.27 to −0.38, indicating a moderate effect (Cramp and Byron-Daniel 2012; Puetz and Herring 2012). Beneficial effects of exercise have been observed in trials conducted with patients during and after treatment, indicating that exercise can be helpful at different stages of the disease trajectory. Aerobic exercise regimens seem to be particularly beneficial. Guidelines from the American College of Sports Medicine (ACSM) recommend that cancer patients and survivors engage in at least 150 min of moderate intensity aerobic activity each week, consistent with recommendations for the general population (Schmitz et al. 2010). ACSM guidelines further recommend that exercise should be tailored to the individual cancer patient to account for exercise tolerance and specific diagnosis, and that patients be closely monitored to safely progress exercise intensity and avoid injury.
Psychosocial interventions are also effective in reducing fatigue, particularly interventions that provide education about fatigue and contributing factors (e.g., physical activity, sleep disturbance) and address dysfunctional fatigue-related thoughts and behaviors. Among women undergoing radiation or chemotherapy for breast cancer, individualized educational and cognitive-behavioral approaches that specifically targeted fatigue buffered the increase in fatigue observed among control patients (Montgomery et al. 2009, 2014; Yates et al. 2005). A brief psychoeducational intervention that provided information about fatigue and modeled adaptive coping strategies (e.g., physical activity) also led to reductions in fatigue among women who had recently completed breast cancer treatment (Stanton et al. 2005). More intensive and targeted treatments have shown benefit for survivors with severe and persistent post-treatment fatigue. These include individual cognitive-behavioral therapy focused on perpetuating factors for persistent fatigue (Gielissen et al. 2006), and a web-based, tailored education program providing information on cancer-related fatigue as well as energy conservation, physical activity, sleep hygiene, distress management, nutrition, and pain control (Yun et al. 2012). Mind-body interventions have also demonstrated efficacy for treating cancer-related fatigue in cancer survivors (see Table 5.2). In particular, specialized programs of acupuncture (Molassiotis et al. 2012), yoga (Bower et al. 2012), and mindfulness (van der Lee and Garssen 2012) led to significant reductions in fatigue among survivors with persistent post-treatment fatigue.
Table 5.2
Randomized controlled trials of mind-body interventions using cancer-related fatigue as an entry criteria
Author, publication date | Participants | Intervention type | Intervention duration | Control group(s) | Results |
|---|---|---|---|---|---|
Bower (2011) | 31 breast cancer survivors with moderate to severe fatigue | Iyengar yoga; group format; focused on postures thought to be effective for reducing cancer-related fatigue (restorative poses, supported back bends, supported inversions) | 12 weeks, 2 sessions per week | Health education group | Decrease in fatigue in yoga group vs. controls at post-intervention; group differences maintained over 3 month follow-up |
Johns (2014) | 35 cancer survivors with moderate to severe fatigue (85.7 % breast) | Mindfulness-based stress reduction; group format; provided training in mindfulness meditation and psycho-education about cancer-related fatigue | 7 weeks, 1 session per week | Wait list | Decrease in fatigue in mindfulness group vs. controls at post-intervention; group differences maintained over 1 month follow-up |
Molassiotis (2012) | 302 breast cancer survivors with moderate to severe fatigue; all post- chemotherapy | Acupuncture; individual sessions; needled 3 standardized points | 6 weeks, 1 session per week | Usual care (fatigue information booklet) | Decrease in fatigue in acupuncture group vs. controls at post-intervention |
van der Lee (2012) | 100 cancer survivors with severe fatigue (58 % breast) | Mindfulness-based cognitive therapy; group format; provided training in mindfulness meditation and using mindfulness to manage automatic negative thoughts about fatigue | 9 weeks, 1 session per week | Wait list | Decrease in fatigue in mindfulness group vs. controls at post-intervention; improvement maintained over 6 month follow-up |
In terms of pharmacologic interventions, there is mixed evidence for the effectiveness of psychostimulants (e.g., methylphenidate) and other wakefulness agents (e.g., modafinil) as treatments for cancer-related fatigue (Minton et al. 2008, 2011). Several large trials of these agents have yielded negative effects, though subgroup analyses suggested that patients with severe fatigue may show some benefit (Jean-Pierre et al. 2010; Moraska et al. 2010). However, there is very limited evidence of their effectiveness in reducing fatigue in patients who are disease free following active treatment. American ginseng may hold promise for treating cancer-related fatigue, particularly among patients undergoing treatment, but more research on this agent is needed (Barton et al. 2013). Of note, very few of the pharmacologic trials have focused specifically on breast cancer patients or survivors.
Interventions for Cognitive Complaints
There have been relatively few studies designed to provide intervention for cognitive dysfunction in cancer survivors, and most of them have been conducted in breast cancer. The first study by Ferguson et al. (2007) was a single arm, individually delivered cognitive behavioral therapy (CBT) approach to memory problems. Due to feasibility and improvements in objective and subjective evaluation, this was expanded to a phase II randomized wait-list controlled trial (Ferguson et al. 2010) that showed trends towards improvement in some aspects of quality of life and memory, but was not definitive. We recently conducted a pilot feasibility trial of a 5 week, group intervention, cognitive rehabilitation program adapted from strategies used in older adults with mild cognitive impairment (Ercoli et al. 2013). This single arm study in 27 breast cancer survivors demonstrated feasibility as well as improvement in self-report and neurocognitive testing up to 6 months post intervention. A small sub-study showed significant normalization of EEG patterns in women who participated in the intervention. Recently, we completed a phase II randomized controlled trial of the same intervention compared to a wait-list control group, and showed highly significant improvements in self-report, neurocognitive tests, and EEG in the intervention group compared to the control group, which was sustained out to 2 months post-intervention, along with improvements in EEG correlating with those who had improved cognitive complaints (Ercoli et al. 2015). These very encouraging findings suggest there is a physiological basis for the improvement in cognitive complaints and test performance.
Other groups have applied computerized technologies to improve cognitive function in breast cancer patients. Kesler et al. (2013a) in a pilot study which randomized 41 breast cancer survivors to a computerized training program focused on executive functioning and memory found significant improvements in those who received the training compared to those who did not. Von Ah et al. (2012) examined a computer-based memory or processing speed training program compared to a wait-list control group of breast cancer survivors. They found that the processing speed training improved that outcome and memory immediately post-intervention and 2 months later. The memory training improved memory performance on neuropsychological testing.
There has also been exploration of psychostimulants to improve fatigue (Jean-Pierre et al. 2010) and secondarily cognitive function, but the findings are not conclusive (Kohli et al. 2009). Other investigators have attempted to examine methylphenidate without success, in terms of adequate recruitment to a treatment trial (Mar Fan et al. 2008). Any such therapy would have to have minimal side effects if it is given chronically, and many breast cancer survivors are averse to continue taking medication long-term if it is not truly necessary or very helpful. Thus behavioral strategies have greater appeal.
What Are the Research Challenges Associated with These Two Common Symptoms?
One of the critical challenges in the area of cancer-related fatigue and cognitive dysfunction is determining the underlying mechanisms for these symptoms. Although cross-sectional research has shown a positive association between inflammatory activity and fatigue in cancer patients and survivors, the causal nature of this association has not been determined. In particular, it is unknown whether inflammation causes fatigue (as observed in experimental models of sickness behavior), or whether inflammation is a consequence of fatigue (perhaps due to reductions in physical activity, alterations in sleep, or other behavioral/physiological changes). One challenge to advancing research in this area is the lack of animal models of cancer-related fatigue (Dantzer et al. 2012). To directly address the causal role of inflammation in a human model, we conducted a small pilot study to evaluate the acute effects of infliximab, a monoclonal antibody against TNF, in five breast cancer survivors with severe, persistent fatigue. Participants completed daily diaries for 2 weeks before and after receiving a single dose of infliximab to assess changes in the severity and duration of daily fatigue. All five women reported reductions in daily fatigue, including a mean 1.9 point decrease in “worst” fatigue from pre- to post-treatment. These preliminary findings are promising and could be pursued in a larger randomized, placebo-controlled trial to determine the causal role of inflammation in cancer-related fatigue. However, anti-cytokine therapies have well-known side effects that may limit their use among women with breast cancer. In addition, given the multi-factorial nature of fatigue, it is likely that only certain women will respond to these (or other) anti-inflammatory agents. Indeed, a recent trial of infliximab for depression found that only those patients with elevated inflammation at treatment onset showed a positive response to this medication (Raison et al. 2013). Similarly, only patients with elevated inflammation are likely to show reduced cancer-related fatigue (and improvements in cognitive function) following anti-inflammatory therapies. Patients whose fatigue is driven by cognitive processes, such as catastrophizing, may be more responsive to cognitive-behavioral therapies, whereas those fatigue is driven by deconditioning may be responsive to exercise. Of course, these treatments may have multiple targets; for example, in our yoga trial with fatigued breast cancer survivors, women in the intervention group reported higher self-efficacy to manage fatigue symptoms and lower inflammatory activity, both of which may have contributed to their reduced fatigue (Bower et al. 2012, 2014). Identifying appropriate treatments for individual patients is an important challenge for future research. In addition, determining the factors that influence fatigue onset vs. persistence may be helpful in determining which type of interventions may be most helpful during vs. after treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree