Neuroendocrine tumors of the small bowel are rare, slow-growing malignancies that commonly metastasize to nodes at the root of the mesentery and the liver. Liver metastases are associated with carcinoid syndrome. Mesenteric nodal masses can cause bowel obstruction, intestinal angina, or variceal hemorrhage. Patients die of liver failure or bowel obstruction. Primary resection is associated with improved survival rates. Selected patients may benefit from liver debulking operations. Liver resection has excellent survival rates even in the event of an incomplete resection, as well as improvement in hormonal symptoms. Radiofrequency ablation can help to preserve hepatic parenchyma during resection.
Key points
- •
Primary tumors tend to remain small and have metastasis, which is different than the typical cancer paradigm.
- •
Resection of the primary tumor may be associated with improved survival rates, even in the setting of unresectable metastases.
- •
Tumors tend to metastasize to nodes at the root of the mesentery, resulting in bowel obstruction and ischemia to vital organs. Aggressive resection should be considered.
- •
Patients with neuroendocrine liver metastases may benefit from an aggressive surgical approach even if they do not receive a complete resection and have extrahepatic disease.
- •
Radiofrequency ablation is an important adjunct to resection and can be used to help preserve hepatic parenchyma or in patients who are not surgical candidates.
Introduction: nature of the problem
Neuroendocrine tumors of the small bowel (carcinoids) are rare and slow-growing tumors that arise from neuroendocrine cells in the gastrointestinal tract. They have a high propensity to metastasize to nodes at the root of the mesentery and to the liver. They are also capable of producing hormones that, when released from the liver, cause carcinoid syndrome of flushing, diarrhea, and congestive heart failure. Approximately 80% of patients who die of disease die of liver failure, with 16% dying of bowel obstruction. Surgical treatment can have a significant impact on these outcomes as well as relieving symptoms and improving quality of life.
Introduction: nature of the problem
Neuroendocrine tumors of the small bowel (carcinoids) are rare and slow-growing tumors that arise from neuroendocrine cells in the gastrointestinal tract. They have a high propensity to metastasize to nodes at the root of the mesentery and to the liver. They are also capable of producing hormones that, when released from the liver, cause carcinoid syndrome of flushing, diarrhea, and congestive heart failure. Approximately 80% of patients who die of disease die of liver failure, with 16% dying of bowel obstruction. Surgical treatment can have a significant impact on these outcomes as well as relieving symptoms and improving quality of life.
The alternate tumor paradigm of neuroendocrine tumors
Most physicians are trained in the standard cancer paradigm. This paradigm can be represented as a pyramid structure ( Fig. 1 ). The cancer originates in the primary tumor, represented by the bottom layer of the pyramid. The primary tumor has to grow to a substantial size before it spreads to the next level, which is lymph node metastases. The volume of the lymph node metastases is expected to remain smaller than that of the primary tumor. There has to be a large primary tumor and a high number of positive lymph nodes before we expect the disease go to the next level, which is distant metastases, represented by the capstone of the pyramid. This is the entire basis of the tumor–nodes–metastases (TNM) staging system. Thus, when patients present with metastatic disease, we expect the primary tumor to be large and easily detectable on physical examination, imaging, or endoscopy. In the unusual cases when it is not, the primary tumor is considered occult and unlikely to ever be found.
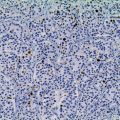
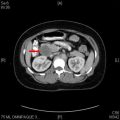
Small bowel neuroendocrine tumors follow a different paradigm, represented by an upside-down pyramid ( Fig. 2 ). The majority of patients present with numerous liver metastases ( Fig. 3 ). Imaging searches for the primary tumor find at most a very large nodal mass at the root of the mesentery with surrounding desmoplastic reaction ( Figs. 4 and 5 ), represented by the middle layer of the pyramid. Ironically, the primary tumor that produced such voluminous metastatic liver and nodal disease is still so small ( Fig. 6 ), represented by the tip of the upside-down pyramid, that it eludes detection on virtually all localizing tests and examinations. When the search fails to find a primary tumor, physicians subscribing to the standard cancer paradigm conclude that the primary tumor is in fact occult and can never be found. However, with the neuroendocrine tumor paradigm, the primary tumor is actually expected to be small. Importantly, this does not imply that it is truly occult and unable to ever be found.
Successfully locating “occult” primary neuroendocrine tumors
Our institution published a series of 63 patients presenting with metastatic neuroendocrine tumors. These 63 patients were evaluated with a total of 177 computed tomography scans, MRI scans, gastrointestinal contrast studies, scintigraphy with radiolabeled octreotide (OctreoScans, Mallinckrodt Inc, St. Louis, MO), endoscopies, and capsule videography to search for their primary tumors. Only 6.2% of these tests correctly located a primary tumor. Unfortunately, this true-positive rate was confounded by a false-positive rate nearly as high at 4.7%. However, surgical exploration found the primary tumor in 80% of cases. Eighty percent of the successfully located tumors were found laparoscopically. If tumors were not visualized laparoscopically, the periumbilical incision for the laparoscope was enlarged slightly and the small bowel was eviscerated a few centimeters at a time through that incision, palpated, and replaced. This resulted in finding additional primary tumors not visible by laparoscopy. All tumors would have been found without preoperative knowledge of their location.
Primary tumor resection
With most other types of cancer, resecting a primary tumor in the presence of distant metastases does not affect survival, but this may not be the case with small bowel neuroendocrine tumors. One of the first reports of improved outcomes in patients with metastatic disease came from Hellman and colleagues. They reported 314 patients with small bowel neuroendocrine tumors with mesenteric nodal or liver metastases. Patients whose primary tumors were resected had significantly longer median survival of 7.4 years compared with 4.0 years for those whose primary tumors were not resected. However, there were 65 patients in the series with no liver metastases who were likely in the primary tumor resected group, whereas most of the patients who did not have their primary tumor resected likely had liver metastases. Therefore, it is possible that some of the difference in survival may be owing to a difference in distribution of patients with liver metastases between the 2 comparison groups.
In 2006, our institution reported a group of 84 patients with small bowel neuroendocrine tumors, all of whom had inoperable liver metastases. Sixty patients had their primary tumors resected and 24 did not. The 2 groups were comparable with respect to all other clinical factors examined. Median time to liver progression was 56 months for patients whose primary tumors were resected compared with 25 months for those whose primary tumors were not resected. Furthermore, the median survival of patients whose primary tumors were resected was 159 months compared with 47 months for those whose primary tumors were not resected. Because, unlike in the series reported by Hellman and associates, all the patients in this series had inoperable liver metastases, the difference in survival cannot be attributed to lack of liver metastases in some of the patients who underwent primary tumor resection.
In 2009, Ahmed and colleagues published a review of 360 patients from the United Kingdom and Ireland with liver metastases from small bowel neuroendocrine tumors. Primary tumor resection was one of several factors associated with improved survival on univariate analysis. More important, primary tumor resection remained a significant predictor of improved survival on multivariate analysis. The authors concluded that primary tumor resection is an independent factor associated with improved survival in patients with liver metastases.
These data are retrospective and may have been influenced by selection bias and/or referral bias, and it is highly unlikely that randomized prospective data will be forthcoming in the foreseeable future. However, based on the findings of these 3 independent series, we recommend operative exploration to locate and resect primary small bowel neuroendocrine tumors, even among patients with unresectable liver metastases, with the minimal goals of relieving symptoms and preventing future complications and bowel obstruction, even though the data on survival benefit are not definitive. Understandably, there may be hesitance to submit patients with unresectable liver metastases to a major abdominal operation to locate their primary tumor when no target has been identified preoperatively and therefore considered unlikely to be successful. However, as demonstrated in the series from our institution, primary tumors can be located successfully surgically in the vast majority of patients despite extensive negative preoperative testing. Furthermore, it can usually be accomplished laparoscopically or through a very small extension of the periumbilical incision used to insert the laparoscope. These techniques quickly identify patients in whom the primary tumor can actually be located before proceeding with a larger operation. Furthermore, the few patients in whom the primary tumor could not be located all went home the same day with only a small periumbilical incision.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree