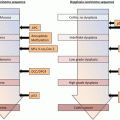
Fig. 6.1
Surgical options for ulcerative colitis patients with dysplasia found on colonoscopy. IPAA ileal pouch-anal anastomosis, IRA ileorectal anastomosis, TAC total abdominal colectomy, TPC total proctocolectomy
6.3.1 Abdominoperineal Total PC
Abdominoperineal total PC is the most definitive treatment for the eradication of undiagnosed synchronous dysplasias and/or carcinomas and the prevention of subsequent metachronous lesions in UC. It allows the resection of synchronous colonic and rectal dysplasia/cancer and avoids the development of metachronous colonic and rectal lesions. It also obviates the need for further colonoscopic surveillance. However, patients must accept a permanent stoma and the postoperative complications are significant, including urinary and sexual dysfunction or a nonhealing perineal wound.
Although this procedure is not an attractive option for patients with comorbidities or for those wanting to preserve anal function, it is indicated for patients with advanced rectal and anal canal cancer or for patients with poor anal sphincter function, such as the older postpartum female. These patients will also require an end ileostomy or a continent Kock ileal pouch.
6.3.2 Segmental/Partial Resection
There is limited debate regarding segmental colectomy in the treatment of lesions in patients with UC in long-standing remission. The indication for the procedures considers the difficulty of further PC and IPAA after lymphadenectomy or adhesions at the target surgical site.
Segmental colectomy is a short operative procedure and maintains continence, but most patients will later require not only further medication but also excision and colectomy and ileostomy. Moreover, a right-sided colo-anal anastomosis is unsuitable for the treatment of left-sided UC [50]. Thus, in general, partial resection of the colon should be avoided because of the high frequency of occult carcinomas and multifocal carcinogenesis. Schwarz et al. [51] reported the case of a patient who underwent a left hemicolectomy and a mucosal proctectomy but then had a macroscopic recurrence of the colitis within 2 months postoperatively and eventually required excision of the remaining colon and an end ileostomy. This suggests that the risk of UC relapse in the residual colon must be taken into account, even if the right side of the colon seems to be in remission.
Patients with UC-associated CRC have a twofold higher mortality than patients with sporadic CRC [52]. Whether segmental or partial resection is the optimal procedure for UC patients with sporadic cancer remains questionable.
6.3.3 Total Abdominal Colectomy with Ileorectal Anastomosis
The cumulative probability of total abdominal colectomy with ileorectal anastomosis (IRA) after 10 years of UC is about 50 %. Total excision was performed following the detection of dysplasia in 5.9 % of these patients [53] and is no longer an acceptable procedure for patients with UC. Although UC is generally considered to always involve the rectum and in some patients also the more proximal portions of the colon, albeit in a diffuse and non-segmental fashion, rectal sparing has been documented [53–57]. If the colitis is totally quiescent or shows rectal sparing, total abdominal colectomy with IRA may be an option for UC patients with a single cancer in the colon. However, since most colectomy specimens with an absence of macroscopic activity show histologic features of chronicity or activity [58], these patients should be monitored for a relapse of proctitis.
The indication of low anterior resection for patients with quiescent UC and rectal cancer or dysplasia should be considered very carefully, because further PC and IPAA would be difficult after this procedure. In addition, patients undergoing total abdominal colectomy with IRA and treated postoperatively with immunomodulators or biologics have a risk of a relapse of inflammation in the residual rectum. Thus, the prognosis, and especially the risk of cancer in the residual rectum, after IRA in patients with UC-associated cancer is a concern. Among patients who received an IRA, regardless of the indication, the estimated cumulative cancer risk after a disease duration of 20 years is 2.1–20.0 % [59–63]. The high long-term risk of cancer after total abdominal colectomy with IRA suggests that this procedure is an interim solution in younger patients. However, IRA with a close follow-up still plays a major role in treating UC patients because it is an easier surgical procedure than IPAA, has excellent functional results, shorter hospitalization, and, importantly, fewer severe complications, unlike in IRA [61].
A particular indication of IRA for UC-associated cancer is a non-obstructing tumor located above the pelvic floor with remote metastases in remission. In the case of an advanced tumor causing obstruction, then a primary colectomy and IRA with a covering ileostomy is advisable.
6.3.4 Subtotal Colectomy
Subtotal colectomy (STC) with end ileostomy and rectal stump pouch are less ideal options because of the retained rectum, which poses a continued cancer risk. However, STC was shown to be a safe treatment option for patients satisfied with an ileostomy or for those with comorbidities that make them ineligible for other procedures or who do not choose to undergo later pelvic pouch surgery. Nonetheless, the potential for the proliferation of residual dysplastic cells or malignant change within the rectal stump in patients who have undergone STC with rectal stump preservation for UC-associated CRC is of serious concern. In addition, whether the potential for malignancy in the rectal stump of STC patients with UC-associated CRC outweighs the morbidity associated with complete proctectomy is difficult to determine. The rate of cancer occurrence later on was shown to be low (1.4 %) in one study [62] but eightfold higher in another [64]. PSC and disease duration until STC were shown to be significant risk factors for rectal stump cancer in a closed rectal stump after STC [65]. Thus, considering the risk of rectal cancer, the low success rate of long-term rectal preservation, and the safety of surgery, a more aggressive approach to early complete proctectomy is recommended in this situation. If this is not possible, patients treated with STC should be followed with close endoscopic surveillance of the closed rectal stump.
6.3.5 Total Proctocolectomy with IPAA
IPAA by ileal J pouch, first described in 1980, is now the gold standard surgical procedure for UC refractory to medical treatment [66]. The long-term quality of life of these patients after this procedure is excellent and the level of fecal continence is satisfactory [67–69]. However, this procedure also has several technical difficulties such as mesenteric lengthening of the pouch [70, 71] and mucosal proctectomy [72]. For surgeons, extensive experience is required to obtain acceptable results [73]. Patients with refractory UC who suffer complications after PC have a poor quality of life [74]. Thus, a double-stapled anastomosis without mucosal proctectomy is the preferred procedure, as there are fewer anastomotic complications and superior rectal continence is achieved; however, a cuff of rectal mucosa is retained, which is the main concern as well as the main argument of opponents of the double-stapling technique. Whether with or without mucosal proctectomy, IPAA is indicated for any colonic or rectal lesion in the surgically fit patient who has unifocal or multifocal dysplasia and refuses a stoma. Relative contraindications of IPAA for UC-associated neoplasias are preoperative incontinence/poor anal sphincter tonus, severe backwash ileitis suggesting Crohn’s disease, and very low rectal or anal dysplasia that threatens the sphincters.
However, the use of a stapled anastomosis without mucosal proctectomy in patients with UC-related dysplasia or cancer remains controversial because of the risk of developing synchronous or metachronous neoplasias in the retained anal transitional zone (ATZ) mucosa. Although an association has yet to be reported between dysplasia and any the following: age, sex, preoperative length of disease, use of a double- vs. single-staple technique, or anastomotic distance from the dentate line [75], the risk of cancer can be reduced by ensuring that the minimal length of rectal columnar mucosa is retained. It is therefore recommended that, in carrying out a stapled IPAA, the anastomosis is performed at the anorectal junction, about 1–1.5 cm above the dentate line, because of the deterioration in anorectal function [76]. Additionally, this procedure is indicated for patients with UC and right-sided colon cancers who require lymph node dissection along the superior mesenteric vein and excision of the marginal arcade of the ileocolic artery, because an insufficient extension of the ileal pouch to the anus precludes a hand-sewn IPAA with mucosectomy.
Stapled IPAA has also been advocated in patients with UC associated with coexisting neoplasia [77, 78]. In these cases, long-term surveillance to monitor dysplasia is recommended; if repeat biopsy confirms persistent dysplasia, ATZ excision with a neoileal pouch-anal anastomosis should be performed [78]. However, restorative PC with mucosectomy does not necessarily eliminate the risks, as after this procedure cancer can occur in a residual ATZ [79, 80]. Thus, in patients with long-standing ileal pouches even after mucosectomy of ATZ, and especially in cases in which dysplasia or cancer is detected in the PC specimen, routine long-term endoscopic surveillance is recommended.
There are many reported cases in the indexed medical literature of carcinoma arising after stapled IPAA for UC [79]. In some studies, the incidence of dysplasia in the ATZ at the time of total colectomy was 2.5–5 %, and duration of UC and patient age at colectomy were significant risk factors [81, 82]. In these cases, mucosal proctectomy is the definitive procedure for patients with preoperatively detected dysplasia in the ATZ.
The incidence of dysplasia after stapled IPAA is 3.0–4.5 % [75, 76, 83]. The development of cancer in the ATZ after stapled IPAA without mucosectomy has been reported [76, 84, 85] and was shown to be significantly associated with a preoperative pathologic diagnosis of UC with concurrent dysplasia or cancer [75]. Based on these data, mucosal proctectomy and hand-sewn IPAA are strongly recommended for patients with neoplasia, especially those with cancer or HGD outside the ATZ [75, 86].
For the reasons stated above, in young patients with UC-associated cancer, mucosal proctectomy with IPAA is recommended, whereas for older patients, particularly those with lower rectal cancer who will accept a permanent stoma, total PC may be proposed. Patients older than 50 years have a significantly higher rate of concurrent dysplasia and malignant degeneration than younger patients, probably because of a longer duration of disease [87]. In these cases, restorative PC with mucosal proctectomy may reduce this risk by eliminating all of the colorectal mucosa.
Branco et al. [88] reported a case in which adenocarcinoma arose in an ileal pouch after IPAA with mucosal proctectomy performed using a cavitron ultrasonic surgical aspirator (Excel, Covidien, Boulder, CO) for UC. This method was introduced to simplify and optimize IPAA with mucosectomy and has been shown to shorten the operative time and reduce blood loss [89]. Its use, however, may increase the number of pathology specimens made uninterpretable on account of tissue ablation. Another ultrasonically activated scalpel (Harmonic; Ethicon Endo Surgery, Johnson & Johnson Medical SPA, Somerville, NJ) also shortened the operative time, decreased blood loss, and was shown to be useful for restorative PC [72]. There has been no report of adenocarcinoma arising in an ileal pouch after mucosectomy performed using this device.
6.3.6 Endoscopic Resection
The ALMs seen in UC patients are similar to those observed in non-UC patients that have been treated by standard polypectomy. This method is associated with little risk of subsequent malignancy on follow-up [42, 44, 90, 91].
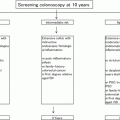
An accurate pathologic diagnosis is very important for distinguishing among the different pathologic entities, given the different therapeutic consequences, such as endoscopic polypectomy for ALM and potential PC for DALM. New and emerging endoscopic imaging techniques, such as chromoendoscopy, magnification endoscopy, and confocal laser endomicroscopy, provide a more accurate diagnosis. Endoscopic resection of an ALM allows confirmation of the biopsy-based adenoma diagnosis and the exclusion of a DALM [91]. However, the endoscopic resectability of a lesion is more important than whether it is an ALM or a DALM [92]. The basic rules for the detection of neoplasia [93] (Table 6.1) should be taken into account and applied in accordance with international guidelines [95–97].
Table 6.1
Basic rules for detecting neoplasia in patients with UC
1. Consult with experienced gastroenterologist |
2. Endoscopic and bioptic control in remission phase |
3. Examination outside routine schedule without time limitation |
4. Ileocolonoscopy with special focus on the detection of DALMs and step (quadrant) biopsies from the rectum to the cecum in 10-cm intervals (sigmoid and rectum: quadrant biopsies at 5-cm intervals) |
5. ALMs with low-grade intraepithelial neoplasia and clear-cut margins can be resected endoscopically |
6. Consult with experienced histopathologist who has all clinical and endoscopy data readily available |
7. Second opinion recommended in cases of histological diagnosis of neoplasia |
Only a few studies have examined the clinical outcomes of DALMs resembling ALMs that are removed with endoscopic polypectomy, but the safety and efficacy of endoscopic resection have been evaluated [93, 94, 98]. Since DALMs, in particular those with a polypoid mass, are an indicator of a high likelihood of the presence of synchronous or metachronous neoplasia, endoscopic resection is not appropriate [22, 40].
6.3.7 Perianal Resection Line for Rectal and Anal Canal Neoplasia
In an IPAA performed in a patient with UC without colorectal neoplasia (Fig. 6.2a.), the mesorectum is resected on the inside, close to the rectal wall, to preserve autonomic nerve function. In patients with UC-associated low rectal neoplasia, total mesorectal excision is required in performing an IPAA (Fig. 6.2b). The choice of operative procedure depends on the depth of the tumor and its distance from the anal verge (Table 6.2). Regarding IPAA with intersphincteric resection, there are no data on postoperative anal function from a large number of cases. Thus, consensus on this procedure is lacking. Depending on curability, however, it should at most be confined to a partial intersphincteric resection (Fig. 6.2b).
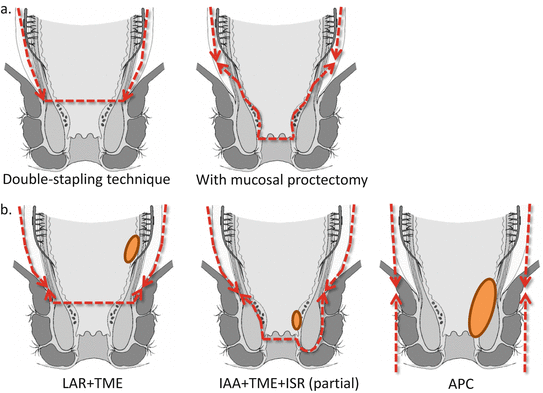

Fig. 6.2
Perianal resection line. Ileal pouch-anal anastomosis (IPAA) for ulcerative colitis (UC) (a), UC-associated low rectal neoplasia (b). TME total mesorectal excision, LAR low anterior resection, IAA ileoanal anastomosis, ISR intersphincteric resection, APC abdominoperineal total proctocolectomy
Table 6.2
Indication and procedure for mesorectal excision in UC patients with rectal and anal canal cancer
Tumor status | Procedure |
|---|---|
T1 | IPAA with mucosal proctectomy (TME is recommended, taking into consideration the risk of a deeper level of T2) |
Deeper level of T2 and not lower than 4 cm from the anal verge | IPAA with mucosal proctectomy and TME |
Deeper level of T2 and lower than 4 cm from the anal verge | IPAA with mucosal proctectomy and TME ± ISR or APC |
Deeper level of T3 or positive for lymph node metastasis | Consider preoperative chemoradiotherapy followed by APC with TME |
6.4 Immunomodulators
Medical therapy for UC has advanced dramatically in the last decade, which has led to discussions of the pros and cons of immunomodulators or biologics for UC patients with malignant disease. Previous studies and guidelines showed that patients administered immunomodulators or biologics do not have a higher risk of new cancer development [99]. Anti-TNF antibodies have been linked to a risk of cancer recurrence in rheumatoid patients, thiopurine to a risk of cancer recurrence in transplant patients, and calcineurin inhibitor to a risk of hepatocellular carcinoma recurrence in liver transplant patients [100–102]. However, a meta-analysis of 74 random controlled trials found that anti-TNF therapies are not related to the short-term clinical emergence of cancer [103]. Nonetheless, a relapse residual lesion is not a rare occurrence after segmental/partial resection; thus, in these patients with advanced cancer, the restricted use of immunomodulators or biologics should be considered.
6.5 Prognosis
6.5.1 Neoplasia in an Ileal Pouch
Previously, cancer of the ileal mucosa was reported in patients who underwent a Brooke ileostomy [104–107] and in those with a Kock pouch [108], but the natural history and prognosis of pouch dysplasia or cancer are poorly understood. Although inflammation, villous atrophy, and colonic metaplasia have been observed within the mucosa of ileal pouches after IPAA, dysplasia may also develop, but the incidence is <0.02 % 20 years after IPAA [109].
In their study of pouch-related adenocarcinoma, Selvaggi et al. [64] showed a pooled cumulative incidence of 0.33 % 50 years after the diagnosis and 0.35 % 20 years after IPAA in a systematic review of the meta-analyses of the literature of pouch-related adenocarcinoma in patients with an IPAA for UC. In that study, one-third of the adenocarcinomas arose from the pouch as a whole and the remainder from the anorectal mucosa [64].
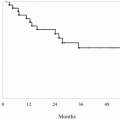
Derikx et al. [110] used the National Registry from 1991 to 2012 to identify 1200 patients with IBD and IPAA; 25 (1.83 %) developed pouch neoplasia, including 16 adenocarcinomas. The cumulative incidence of pouch neoplasia at 5, 10, 15, and 20 years was 1.0 %, 2.0 %, 3.7 %, and 6.9 % for pouch neoplasia and 0.6 %, 1.4 %, 2.1 %, and 3.3 % for pouch carcinoma [110] (Fig. 6.3). A history of colorectal neoplasia was the only risk factor associated with pouch neoplasia. Hazard ratios were 3.76 (95 % CI: 1.39–10.19) for prior dysplasia and 24.69 (95 % CI: 9.61–63.42) for prior carcinoma [110]. Another systematic review similarly concluded that neoplasia in the colectomy specimen was the strongest risk factor (odds ratio = 8.8; 95 % CI: 4.61–16.80) [64].
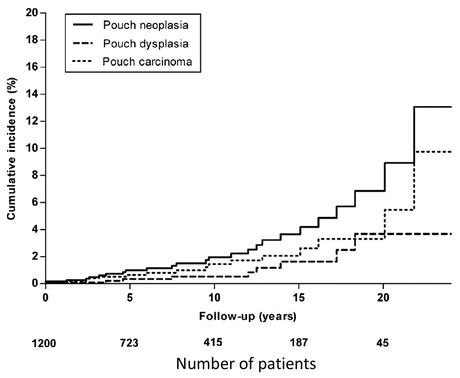

Fig. 6.3
Cumulative incidences of pouch neoplasia (both carcinoma and dysplasia), pouch carcinoma, and dysplasia (From [110])
Malignant transformation of the ileal pouch mucosa may occur even in the absence of backwash ileitis or a previous history of cancer [111, 112], as determined in biopsies from the ileal pouch mucosa obtained at least 1 year after the newly formed pouch that was influenced by fecal flow [113]. Chronic inflammation of the ileal mucosa such as occurs with preoperative backwash ileitis and postoperative pouchitis in UC has also been linked to the sequence of malignant transformation [114–117]. An abnormal lesion of the ileal pouch mucosa was shown to have a high risk of adenocarcinoma 20 years or later after the initial IPAA [118].
PSC-IBD patients are at increased risk of colorectal neoplasia [38], but the development of pouch neoplasia in PSC-UC patients following IPAA is unclear. Imam et al. [119] conducted a retrospective chart review of 65 patients with PSC and IBD who underwent colectomy with IPAA followed by pouch surveillance between 1995 and 2012. The cumulative 5-year incidence of pouch neoplasia was 5.6 % (95 % CI, 1.8–16.1 %). Based on this short-term follow-up, they concluded that a frequent surveillance of the pouch was an unnecessary practice in PSC-IBD patients. However, it is recommended that patients with these risk factors be followed by endoscopy and random biopsies for the rest of their lives. If a pouch-related adenocarcinoma is detected during these examinations, abdominoperineal excision is recommended.
6.5.2 Outcome of Colorectal Cancer in UC
There have been a few reports based on small series that examined the outcome of patients with UC and CRC [12, 120–123]. From a functional aspect, among cancer patients who received an IPAA, no significant differences could be found between those with UC-associated CRC and those with UC without CRC [124]. For UC-associated CRCs, as for non-colitic cancers, histologic stage, site, and mucin content of the tumor are the most important variables determining postoperative survival [49].
A 20-year follow-up study of IBD-related CRC from the Mayo Clinic compared patients with sporadic CRC with age- and sex-matched patients with IBD-related cancers. In the latter group, the tumors were more proximally located, with only 55 % distal to the splenic flexure, compared with 78 % among patients with sporadic CRC [125]. However, compared with sporadic tumors, IBD-related CRC was more often in an advanced stage and more likely to have a mucinous component [125]. Yet, no differences were found in the overall survival of patients with sporadic CRC and those with IBD-related CRC [125].
Heimann et al. [126] showed that the 5-year survival rate was significantly worse for patients with non-diploid tumors (76 % vs. 32 %). When stratified by stage, only patients with Dukes’ C lesions had a significant difference in survival for diploid vs. non-diploid tumors. Multivariate analysis showed that Dukes’ classification was the best prognostic indicator, followed by tumor differentiation and DNA ploidy. Tumor location, colloid content, number of cancers, duration of disease, and patient age and sex did not correlate with the prognosis [126].
A retrospective review of 1642 UC patients by Kavanagh et al. [127] showed that patients who undergo surgery for UC-associated CRC (n = 22) have less favorable short-term outcomes but present at a less advanced stage and have a more favorable long-term prognosis than similar patients with CRC and Crohn’s disease. The overall 5-year survival was significantly better in the UC group than in the group with Crohn’s disease (41 % vs. 29 %; P = 0.04).
Watanabe et al. [128] showed that in a group of 108,536 CRC patients, the 169 with UC-associated CRC had a poorer survival than patients with sporadic CRC (43.3 % vs. 57.4 %; P = 0.0320) for stage III disease but not for early-stage disease. The authors concluded that the detection of UC-associated CRC at an early stage results in similar postoperative outcomes as those of patients with sporadic CRC. A Danish population-based study also compared patients with UC-associated CRC (n = 279) and those with sporadic CRC (n = 71,259). Cancer stage and rates of lymph node and distant metastasis were similar between the two groups, but the overall mortality rates at 1 and 5 years after cancer diagnosis were higher in UC-associated CRC than in sporadic CRC (OR = 1.24; 95 % CI: 1.02–1.51 and OR = 1.17; 95 % CI: 1.01–1.36, respectively) [129]. Other population-based studies showed that patients diagnosed with UC-associated CRC at age <60 years had a worse outcome [130, 131], which, according to Shu et al. [131], was more pronounced in males.
6.5.3 Radiation/Chemotherapy
Although locally advanced rectal cancer requiring multimodality therapy is uncommon in patients with UC, the functional outcome of patients with UC-associated CRC who received adjuvant chemotherapy was shown to be very good if the appropriate surgical technique and chemotherapy protocol were selected [86].
Preoperative chemoradiotherapy (CRT) and total mesorectal excision with or without intersphincteric excision are the current treatment choices for patients with lower rectal cancer. This approach was shown to optimize oncologic outcome and to maintain anorectal function [132]. By contrast, pelvic radiation administered prior to IPAA is associated with poor pouch outcomes for UC patients [86, 133, 134]. In fact, external beam radiation to treat cancer is problematic in UC patients, especially because the small bowel has a lower tolerance than the large bowel [135]. Thus, whether adjuvant CRT increases postoperative complications remains controversial [133, 136].
In patients with cancer located in the ATZ and close to the internal sphincter, restorative PC and partial intersphincteric resection may be indicated [136], whereas preoperative CRT has a negative impact on sphincter function [136–138]. A recent report identified preoperative CRT as a risk factor for impaired anal function after intersphincteric resection [139]. CRT followed by IPAA and partial intersphincteric resection may be even more destructive in terms of postoperative anal function, with several studies showing better outcomes than colonic J pouch reconstruction for lower rectal cancer [140–142]. Previous reports demonstrated a high tolerance of preoperative CRT and pouch surgery with minimum intersphincteric resection [142, 143]. Overall, because prognosis seems to be related to cancer stage, the oncologic benefits and pouch functional outcomes should be carefully balanced before pelvic radiation prior to IPAA is considered [134].
References
1.
Kaplan GG et al (2012) Decreasing colectomy rates for ulcerative colitis: a population-based time trend study. Am J Gastroenterol 107(12):1879–1887PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree