, Daniel Riccioppo1, Flavio Kawamoto1 and Marco Aurelio Santo1
(1)
Bariatric and Metabolic Surgical Unit/Hospital das Clinicas, University of São Paulo, School of Medicine, São Paulo, Brazil
Abstract
The onset of type 2 diabetes is characterized by a nonreversible complex cycle that includes severe deleterious effects on glucose metabolism. Obesity, but mainly visceral adipose tissue accumulation, is an important factor in this process. The goals of diabetes management in clinical practice, despite the improvement over the years, are often not met. In the last 20 years, based on observations of bariatric surgery series that have shown great improvement of type 2 Diabetes in morbid obese patients, metabolic surgery has emerged as a therapeutic possibility. In 2011 the International Diabetes Federation released its position statement mentioning that bariatric surgery was an accepted option for T2DM patients with BMI ≥ 35 kg/m2 and might be considered an alternative therapy for patients with BMI ≤ 35 kg/m2 who do not respond to standard medical therapy. Metabolic/bariatric surgery includes the application of conventional bariatric procedures (Roux-en-Y gastric bypass, biliopancreatic diversion, sleeve gastrectomy) and the introduction of new procedures (ileal interposition, intestinal bipartition) designed with the specific aim of having metabolic effects, irrespective of causing massive weight loss. The reversal of T2DM occurs due to mechanisms such as the increase in insulin sensitivity associated with an improvement in beta-cell function, including recovering the first phase of insulin secretion. This recovery is a consequence of the increase of GLP-1 production, and change in circulating bile acids. Remission of diabetes is observed on the first postoperative days after the operation.
6.1 Introduction: Pathophysiology of Type 2 Diabetes Mellitus
Diabetes Mellitus (DM) is a metabolic disorder characterized by a lack of control of glucose homeostasis resulting in hyperglycemic state, high enough to significantly increase the incidence of a specific type of microangiopathy (retinopathy, nephropathy, and neuropathy). Type 2 diabetes (T2DM) represents 90–95 % of cases of DM. Prediabetes, also known as dysglycemia, is a condition in which blood glucose levels are higher than normal, but not high enough for the diagnosis of diabetes. People may have this condition for several years without noticing anything and before becoming diabetic (American Diabetes Association 2012).
The pathogenic mechanisms involved in the development of T2DM are: (A) peripheral insulin resistance resulting in decreased metabolic responses to insulin and (B) the progressive decline of pancreatic islet cell function resulting in reduced insulin secretion and inadequate suppression of glucagon secretion. Patients with insulin resistance require more insulin to promote glucose uptake by peripheral tissues, and the genetically predisposed ones may lack the necessary β-cell secretory capacity (Carrera Boada and Martinez-Moreno 2013).
Prediabetes can be separated into two different conditions: impaired fasting glucose (IFG), diagnosed by a fasting glucose test, and impaired glucose tolerance (IGT), diagnosed by a postprandial glucose test. The pathophysiology of IFG seems to include the following defects: reduced hepatic insulin sensitivity, stationary β-cell dysfunction and/or chronic low β-cell mass, altered GLP-1 secretion, and inappropriately elevated glucagon secretion. Conversely, the prediabetic state of isolated IGT (IGT without IFG) is mainly characterized by reduced peripheral (muscle) insulin sensitivity and a reduced second-phase insulin secretion. Individuals developing combined IFG/IGT exhibit severe defects in both peripheral and hepatic insulin sensitivity, as well as a progressive loss of β-cell function (Bergman et al. 2002). In conclusion, the transition from the prediabetic states to type 2 diabetes is characterized by a nonreversible vicious cycle that includes severe deleterious effects on glucose metabolism. Obesity, but mainly visceral adipose tissue accumulation, is an important factor in this process. Many epidemiologic studies have shown that body mass index (BMI) is a powerful predictor of type 2 diabetes. Visceral fat is an important source of inflammatory cytokines such as TNF-alpha, TGF-beta, IL6, resistin, and PAI-1 that can directly affect insulin-mediated glucose uptake (insulin resistance). On the other hand, there is a reduction of secretion of other factors such as adiponectin that reduces insulin resistance (Carrera Boada and Martinez-Moreno 2013; DeFronzo 1997). This imbalance leads to a pro-inflammatory state which is related to an increased risk of cardiovascular complications. Along insulin resistance, β-cell secretory function and β-cell mass play complementary roles in the development of type 2 diabetes. This process includes islet amyloid deposits and increased β-cell apoptosis. Besides, abnormal α-cell function (glucagon secretion) is an important determinant of the magnitude of the hyperglycemia found in diabetes, and lipotoxicity characterized by an increase in circulating free fatty acids (FFAs) may contribute to progressive β-cell failure (β-cell lipotoxicity) in individuals genetically predisposed to T2DM (Carrera Boada and Martinez-Moreno 2013; Boden 1997). Another important factor in the physiopathology of the disease is related to the incretin secretion profiles. To date, only glucose-dependent insulinotropic polypeptide (GIP) and glucagon–like peptide 1 (GLP-1) fulfill the definition of an incretin hormone in humans. GIP is secreted by K cells and released from the proximal small intestine (duodenum and jejunum) and GLP-1 is secreted by L cells located predominantly in the ileum but also in the proximal gut. Incretins bind to specific membrane receptors in β cells, enhancing the release of insulin. The profiles of these two hormones are altered in T2DM patients. While GIP concentration is normal or modestly increases in patients with T2DM, the insulinotropic actions of GIP are significantly diminished. In contrast to GIP, the secretion of GLP-1 has been shown to be deficient in patients with T2DM (Carrera Boada and Martinez-Moreno 2013).
6.2 Diagnosis and Results of Clinical Treatment of DM
The diagnosis criteria of DM, according to the American Diabetes Association (ADA), are (American Diabetes Association 2012):
Glycated Hemoglobin greater than 6.5 %
Or fasting glycemia greater than 126 mg/dL
Or glycemia greater than 200 mg/dL, 2 h after the glucose tolerance test
The test should be performed as described by the WHO (World Health Organization), using a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water.
In a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glycemia greater than 200 mg/dL (11.1 mmol/L) is considered diagnostic.
A useful method of measuring insulin resistance and β-cell function is a mathematical model that combines the measurement of fasting blood glucose and insulin. This method is called HOMA and based on it two other indices are extracted (HOMA-IR and HOMA-beta), which aim to translate insulin sensitivity and secretory capacity of β cells. The reference value of the HOMA-IR index for diagnosis of insulin resistance may be preselected, or calculated as the 95th percentile in a healthy population. In the BRAMS study (Brazilian Metabolic Syndrome Study), the cutoff point was 2.71 (Malerbi and Franco 1992).
The dosage of hemoglobin A1c became increasingly used and accepted by the scientific community after 1993, after being validated by two major clinical studies assessing the impact of glycemic control on chronic complications of diabetes: the DCCT study—Diabetes Control and Complications Trial, and UKPDS—United Kingdom Prospective Diabetes Study (Smyth-Osbourne et al. 1998; Murray et al. 2010). A1c reflects serum glucose levels 2–3 months prior to its measurement. In a nondiabetic individual, approximately 4–6 % of A1c is observed, while in the uncontrolled diabetic this percentage may reach levels two to three times above normal. A1c levels above 7 % are associated with a progressively higher risk of chronic complications. Therefore, current diabetes treatment goals set 7 % as the upper limit. If A1c is above 7 %, revision of the therapeutic regimen is indicated (Zachary and Bloomgarden 2010).
The treatment of type 2 diabetes and its complications focuses on control of glucose levels, initially based on diet, encouraging physical activities and losing weight, and oral hypoglycemic drugs (sulfonylureas, meglitinides, biguanides, pioglitazone, and DPP-4 inhibitors). GLP-1 analogues (exenatide or liraglutide) are used subcutaneously and may increase insulin secretion. Along the evolution of the disease and the impairment of insulin secretion by pancreatic β cells, patients may need insulin therapy.
The large clinical trials such as UKPDS (United Kingdom Prospective Diabetes Study), DCCT/EDIC (The Diabetes Control and Complications Trial and Follow-up Study), and ADVANCE (Action in Diabetes and Vascular Disease) (Murray et al. 2010; The ADVANCE Collaborative Group 2008) have proven the importance of the early intensive control of diabetes, to prevent complications of the disease, and to improve the quality and quantity of life of these patients, bringing the concept of “metabolic improvement.”
A strategy of intensive glucose control to lower the hemoglobin A1c (HbA1c) value to 6.5 % yielded a 10 % relative reduction in major macrovascular and microvascular events (The ADVANCE Collaborative Group 2008).
On the other hand, more intensive glucose control in patients with poorly controlled T2DM had no significant effect on the rate of major CV events, microvascular complications, or death, due to complications related to hypoglycemia episodes (The VADT Investigators 2009).
In the same way, in the ACCORD study, high-risk T2DM patients submitted to intensive therapy to lower HbA1c had an increased mortality and no significant reduction in major CV events, as compared to standard medical therapy (ACCORD Study Group 2008).
The goals of diabetes management in clinical practice, despite the improvement over the years, are often not met. A Brazilian study published in 2010 showed only 27 % of control (glycated hemoglobin HbA1c—less than 7 %) in about 6,000 type 2 diabetic patients (Mendes et al. 2010); in the same way, a recent U.S. publication reported that 52.5 % of 1,343 patients with T2DM had HbA1c < 7, and only 18.8 % achieved the goal of multifactorial control (HbA1c < 7, LDL < 100 mg/dL, and less than 130 × 80 mmHg of blood pressure) (Starck Casagrande et al. 2013).
6.3 The Role of Surgical Treatment of Type 2 Diabetes
From the moment that the objectives of the clinical treatment of type 2 diabetes are not being achieved, despite the use of all clinical resources (medications, diet, change of lifestyle), metabolic surgery emerges as a therapeutic possibility. In the last 20 years, based on observations of bariatric surgery series, several articles have investigated whether type 2 DM (T2DM) could be a disease of the foregut (Hickey et al. 1998; Rubino and Marescaux 2004; Pories et al. 2011). The nonrestrictive and nonmalabsorptive effects of bariatric surgery became a topic of interest, and the possibility of using bariatric surgical procedures to treat T2DM as the prime objective (metabolic surgery) became a goal among bariatric surgeons.
But the concept of metabolic surgery was defined earlier, by Buchwald and Vanco in 1978 in their book “Metabolic Surgery”, as the operative manipulation of the normal organ or organ system, to achieve a biological result for a potential health gain (Buchwald and Varco 1978). Now, metabolic surgery is defined as any modification of the gastrointestinal (GI) tract, where rerouting the food passage seems to improve T2DM, based on mechanisms that are weight loss independent. In this way, bariatric surgery has emerged as a potentially useful treatment for T2DM (Deitel 2011). Observational studies and randomized controlled trials have shown that procedures including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), gastric banding (GB), and biliopancreatic diversion (BPD) significantly improve glycemic control, and favorably affect cardiovascular risk factors (Buchwald et al. 2009; Schauer et al. 2003; Dixon and O’Brien 2002). The remission rate of T2DM varies with the surgical procedure, and best results are seen in operations that include a bypass of the gastrointestinal tract (RYGB, BPD) when compared to those obtained with the purely restrictive operations (GB), in which remission is directly associated with weight loss (Table 6.1).
Table 6.1
Studies evaluating outcomes of DM control with different surgical procedures
Studytype | Type II diabetes | BMI | Follow-p | Surgery 1 | Surgery 2 | Outcome | Result with 1 (%) | Result with 2 (%) | |
|---|---|---|---|---|---|---|---|---|---|
Schauer et al. (2014) | Randomized controlled trial | Yes | >35 | 3 years | RYGB (Koch and Finelli 2010) | Sleeve gastrectomy (Shimizu et al. 2012) | HBA1C < 6 % | 38 | 24 |
Mingrone et al. (2012) | Randomized controlled trial | Yes | >35 | 2 years | RYGB (Deitel 2011) | BPD (Deitel 2011) | HBA1c < 6 %, normal fasting plasma glucose, and off oral hypoglycemics | 75 | 95 |
Vidal et al. (2008) | Prospective | Yes | >35 | 1 year | RYGB (Pournaras et al. 2012) | Sleeve gastrectomy (Laferrere et al. 2008) | HBA1c < 7 % and normal fasting plasma glucose | 87 | 87 |
Pinheiro et al. (2008) | Randomized controlled trial | Yes | >50 | Mean 4 years | RYGBa (Kota et al. 2012) | RYGBb (Karamanakos et al. 2008) | Normal glycemia and off oral hypoglycemics | 58 | 93 |
Pournaras et al. (2010) | Prospective | Yes | >35 | 3 years | RYGBa (Murray et al. 2010) | LAGB (The ADVANCE Collaborative Group 2008) | HBA1c < 6 %, normal fasting plasma glucose, and off oral hypoglycemics | 72 | 17 |
This new frontier of metabolic/bariatric surgery includes the application of conventional bariatric procedures (RYGP, BPD, SG) and the introduction of new procedures (ileal interposition, intestinal bipartition) designed with the specific aim of having metabolic effects, irrespective of causing massive weight loss.
In view of growing enthusiasm for surgical interventions to treat T2DM, the 1st Diabetes Surgery Summit was held in Rome on March 2007 to develop guidelines to use GI surgery to treat T2DM. In 2009 the American Diabetes Association first mentioned surgical therapy for treating T2DM. In 2011 the International Diabetes Federation released its position statement mentioning that bariatric surgery was an accepted option for T2DM patients with BMI ≥ 35 kg/m2, and might be considered an alternative therapy for patients with BMI ≤ 35 kg/m2 who do not respond to standard medical therapy (Dixon et al. 2011).
Shimizu et al. have reviewed papers about metabolic surgery for T2DM patients with BMI < 35 kg/m2, and found that most of the patients achieved normal BMI after surgery and only 2.7 % reported excessive weight loss with no evidence of malnutrition. Discontinuation of antidiabetic medication and remission of T2DM were achieved in 86.8 and 64.7 % of the patients, with FPG and HA1c at slightly above normal range. Moreover, metabolic surgery provided adequate glycemic control for 30.1 % of the patients using insulin prior to surgery (Shimizu et al. 2012).
It has been described that malabsorptive bariatric procedures have higher diabetes remission rates than restrictive ones (Buchwald et al. 2009; Scopinaro et al. 2005). Nevertheless, in the SOS study, where most surgical procedures were of the purely restrictive type, the average weight loss after 10 years was 25 % in the surgical group, compared to the control group (clinical treatment), where a weight gain of 1.6 % was observed. The risk of developing diabetes over the years has been 3× higher in the control group (Sjostrom et al. 2004). This information points to an old concept: the importance of losing weight (mainly visceral fat) as a key mechanism of T2DM control in obese patients, no matter how it is achieved (Larsson et al. 1981; Everhart et al. 1992).
6.4 Surgical Techniques
6.4.1 Current Bariatric Procedures
The technical procedure known as Roux-en-Y gastric bypass (RYGP) was endorsed as a standard in the surgical treatment of severe obesity (NIH Conference Gastrointestinal Surgery for Severe Obesity 1991). It is the mostly performed bariatric procedure in the world, and is considered the gold standard for surgical treatment of morbid obesity in the USA (Buchwald and Oien 2013).
RYGP (Fig. 6.1) is considered a combined procedure, i.e., it promotes weight loss by restricting food intake and also generates, although in small proportion, intestinal malabsorption of nutrients.
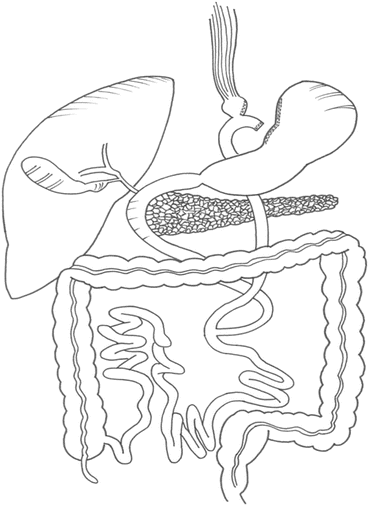

Fig. 6.1
Roux-en-Y gastric bypass (Image supplied by Dr. Daniel Riccioppo)
It consists in configuring a gastric pouch with a volume of about 15–30 cc, with or without the placement of a band for restriction of flow, and restoration of intestinal transit with a Roux-en-Y intestinal bypass 1.2–2.2 m long in average. About 50–100 cm is the length of the biliopancreatic limb, which leads digestive secretions to the entero-anastomosis, and 70–120 cm of the alimentary limb, which takes the bolus from the gastric pouch to the entero-anastomosis. This prevents the bypassed duodenum and first portion of jejunum to receive the alimentary bolus, which reaches more quickly the distal jejunum and the ileum, causing hormonal responses that may explain the surgical effects on glycemic control.
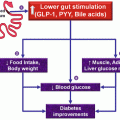
After RYGP, the following results can be observed, with important metabolic effects: gastric restriction, leading to early satiety, and decreasing the volume of the meals; exclusion of the fundus of the stomach from the alimentary transit, leading to a reduction in the secretion of ghrelin and subsequent anorexigenic effect; and faster arrival of nutrients to the distal intestine, in order to stimulate the release of PYY and GLP-1, which lead to decreased food intake and improve glucose tolerance (Rubino et al. 2006; Cummings et al. 2007).
The reversal of T2DM occurs due to an increase in insulin sensitivity, associated with an improvement in beta-cell function, including recovery of the first phase of insulin secretion. This recovery is due to an increase of GLP-1 production. Remission of diabetes is observed on the first postoperative days after RYGP (Laferrere et al. 2008).
This early antidiabetic effect is not observed in patients undergoing purely restrictive procedures, such as adjustable gastric banding. This finding reinforces the role of enterohormones in metabolic effects of bariatric procedures, and not just consequence of weight loss.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree