Surgery remains a mainstay in the management of localized prostate cancer. This article addresses surgical aspects germane to the management of men with prostate cancer, including patient selection for surgery, nerve-sparing approaches, minimization of positive surgical margins, and indications for pelvic lymph node dissection. Outcomes for men with high-risk prostate cancer following surgery are reviewed, and the present role of neoadjuvant therapy before radical prostatectomy is discussed. In addition, there is a review of the published literature on surgical ablative therapies for prostate cancer.
Key points
- •
An individual’s health state, not absolute age, should be utilized when determining fitness for radical prostatectomy.
- •
When performing a pelvic lymph node dissection for prostate cancer, an extended lymph node dissection should be performed.
- •
Nerve sparing selection should not compromise the oncologic goal of complete excision of the tumor with negative surgical margins.
- •
Multimodal therapy is often required for men with high risk prostate cancer.
Introduction
Prostate cancer (PCa) is the most common cancer diagnosis in men, with 238,590 estimated cases diagnosed in 2013. PCa incidence increases with age, with the highest rates seen in men aged 70 to 80 years. Despite the low PCa case-fatality rate, the established overdiagnosis attributed to prostate-specific antigen (PSA) screening, and the fact that most men with PCa will die of other causes, the majority of men still opt for surgical management of newly diagnosed PCa. This article addresses various surgical and ablative approaches to local control of the primary tumor. A structured debate of the relative efficacies of the various treatments is beyond the scope of this article. Rather, here the authors address surgical aspects germane to the management of men with low-risk and high-risk PCa; the role of lymph node dissection (LND), surgical margins, and neoadjuvant therapy in localized PCa; and the content of published literature on surgical ablative therapies for PCa.
Introduction
Prostate cancer (PCa) is the most common cancer diagnosis in men, with 238,590 estimated cases diagnosed in 2013. PCa incidence increases with age, with the highest rates seen in men aged 70 to 80 years. Despite the low PCa case-fatality rate, the established overdiagnosis attributed to prostate-specific antigen (PSA) screening, and the fact that most men with PCa will die of other causes, the majority of men still opt for surgical management of newly diagnosed PCa. This article addresses various surgical and ablative approaches to local control of the primary tumor. A structured debate of the relative efficacies of the various treatments is beyond the scope of this article. Rather, here the authors address surgical aspects germane to the management of men with low-risk and high-risk PCa; the role of lymph node dissection (LND), surgical margins, and neoadjuvant therapy in localized PCa; and the content of published literature on surgical ablative therapies for PCa.
Patient selection
Historically, men with a life expectancy of longer than 10 years and localized disease were considered eligible for radical prostatectomy (RP). However, many clinicians may not accurately predict life expectancy in men with localized PCa. Consequently life-expectancy tables, which provide the median survival for a man at a given age, are often consulted. Because life tables do not take into consideration an individual’s comorbidities, they may not accurately predict overall survival in men with different severities of comorbidity. For example, a 70-year-old man in the healthiest quartile has a life expectancy of more than 18 years, compared with an estimated life expectancy of less than 7 years for a 70-year-old in the lowest quartile health state ( Fig. 1 ).
Nomograms incorporating comorbidities to predict life expectancy in men with localized PCa, which may be more accurate than life tables, are available.
In general, age should not be an absolute contraindication for RP. The National Comprehensive Cancer Network (NCCN) Guidelines for senior adult oncology state that “irrespective of age, a person who is functionally independent and without serious comorbidity should be a good candidate for most forms of cancer treatments.” Furthermore, the International Society of Geriatric Oncology (ISGO) specifically addresses treatment of elderly men with localized PCa, and recommends that healthy elderly men receive standard treatment as for younger men, whereas men with vulnerable health status (reversible health problem) should be offered standard treatment exclusive of RP. Single-institution series of elderly men undergoing RP demonstrate that in carefully selected men, RP can be beneficial. For example, in one RP series of elderly men the 15-year PCa-specific and overall survival was 90.2% and 68.9%, much higher than in population-based series.
Surgical technique
Radical removal of the prostate can be performed using open (radical retropubic or perineal) and minimally invasive (laparoscopic or robotic-assisted) techniques. The primary methods of prostate removal today are either radical retropubic or robotic-assisted, although the perineal approach and pure laparoscopic prostatectomies can provide comparable oncologic and functional outcomes in experienced hands in comparison with the more common approaches. The anatomic points of the retropubic approach were defined by Walsh in 1982, and remain the standard against which all other approaches are compared. Since its first description using the Da Vinci system in 2001, there has been increased adoption of robotic-assisted RP, such that 62% of RPs performed in 2009 were performed robotically. A comparison of oncologic and functional outcomes between radical retropubic prostatectomy and robotic-assisted prostatectomy is beyond the scope of this article. The body of published literature that attempts to compare oncologic and functional outcomes, primarily between open and robotic-assisted approaches, is conflicting and controversial. Although it is well established that minimally invasive approaches are associated with less blood loss, decreased narcotic use, and decreased hospital stay, the primary comparative function outcomes of incontinence and erectile dysfunction are not well characterized, although multiple notable reports have addressed these issues. Similarly, the long-term oncologic outcomes of open versus robotic-assisted methods have not been fully reported, hampering meaningful conclusions regarding the cancer-specific outcomes of open and robotic approaches. A detailed analysis of the “trifecta” (continence, sexual function, and oncologic control) is beyond the scope of this article. In the authors’ opinion it is the surgeon, not the approach, which is most critical to an individual’s outcomes following prostatectomy, a stance that is supported by leaders in both minimally invasive and open surgical approaches.
Cavernosal nerve sparing
The cavernosal neurovascular bundles (NVB) that are responsible for erectile function are located along the posterolateral surface of the prostate, lying directly on the prostatic capsule. Nerve-sparing techniques are frequently used whereby the NVBs are carefully dissected off the surface of the prostate, taking care not to disrupt the prostatic capsule. In the setting of organ-confined disease, the surgeon can safely spare these nerves without compromising oncologic efficacy, provided that no iatrogenic capsular incision is made. The challenge lies in accurately predicting which cancer will be organ-confined disease and which cancers may have microscopic extracapsular extension (ECE) such that nerve-sparing techniques may have an impact on surgical margin rates. Others have advocated wide-field resection of NVBs in the setting of all high-grade or high-volume disease, such as in the setting of high-risk disease.
There are several tools available for predicting the risk of ECE to guide clinicians and patients on whether or not to spare the NVB, primarily predicting the laterality of ECE. These different tools combine a varying number of several different factors: 2 factors (1 positive core with ≥7 mm of tumor and a positive base biopsy) ; 3 factors (Gleason score, % of core with tumor involvement, and perineural invasion ; Gleason score, PSA, and % of cores with cancer ; or Gleason score, PSA, and clinical stage ); and 5 factors (PSA, clinical stage, Gleason score, % of core tumor involvement, % of cores with cancer). Individual surgeons will favor varying risk cutoffs regarding when to attempt nerve-sparing techniques.
The prostatic anatomy lends itself to various approaches of nerve-sparing versus wide resection. The detailed description of the fascial planes allows for variable depths of dissection, as shown in Fig. 2 . For example, intrafascial dissection is in close proximity to the prostatic capsule, and is more likely to be used in low-risk PCa with low probability of ECE. Conversely, an extrafascial dissection is more likely to be used in an individual for whom the risk of ECE is high.
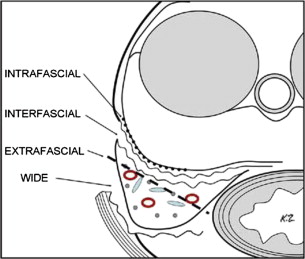
In addition, intraoperative findings can guide the decision on nerve-sparing approaches. Visual inspection or, in the case of open prostatectomy, manual palpation, can be used to help define the proximity of a tumor to the nerves. Intraoperative biopsies can also assist in decisions regarding nerve sparing. Others have promoted emerging technologies to identify the nerves during robotic-assisted laparoscopic prostatectomy. In all cases, the decision to perform nerve sparing should not compromise the oncologic goal of complete excision of the tumor with negative surgical margins.
Pelvic lymph node dissection
There are 2 major issues that surround LND for PCa. First, the boundaries of lymph node dissection are variable. Traditionally, most surgeons perform a pelvic LND (PLND) that is limited to the obturator fossa and external iliac vein. However, the extent of PLND has grown with increasing recognition that the lymphatic drainage of the prostate is linked to the external and internal iliac arteries and the presacral regions. Several reports have found that the limited PLND will miss 20% to 40% of positive lymph nodes. The extent of the extended PLND (ePLND) is yet to be determined. The NCCN guidelines describe the boundaries to be “external iliac vein anteriorly, the pelvic side wall laterally, the bladder wall medially, the floor of the pelvis posteriorly, Cooper’s ligament distally, and the internal iliac artery proximally.” According to the European Association of Urology (EAU) guidelines, regions to be removed include the nodal tissue “overlying the external iliac artery and vein, the nodes within the obturator fossa located cranially and caudally to the obturator nerve, and the nodes medial and lateral to the internal iliac artery.” However, it should be noted that mapping studies have shown that there are additional areas of primary drainage not included in these templates (presacral, perirectal, common iliac).
The therapeutic benefit of ePLND is less well defined. Whereas ePLND certainly provides more accurate staging and will detect a greater number of patients with positive lymph nodes, potentially helping identify adjuvant therapy candidates, it is not clear that it results in improved oncologic outcomes. Some patients with positive nodes experience long-term disease-free intervals, with approximately 10% to 15% of patients remaining without evidence of recurrence at up to 10 years. Several studies have found that a greater number of lymph nodes removed is associated with improved disease-specific outcomes or that in those with positive nodes, a lower lymph node density is associated with improved outcomes. Along these lines, better outcomes have been observed in men with only 1 positive lymph node. However, these findings must be tempered with the higher risk of complications and added operative time with ePLND.
The second major issue surrounds the need and indication for PLND, and most have promoted LND based on probability of lymph node involvement (LNI). For example, the NCCN guidelines recommend a PLND if the probability of LNI is 2% or greater, and states that “an extended PLND will discover metastases approximately twice as often as a limited PLND.” Extended PLND provides more complete staging and may cure some men with microscopic metastasis; therefore, “an extended PLND is preferred when PLND is performed.” The EAU recommends that a PLND be performed if the risk of LNI is 5% or greater, essentially excluding a PLND for men with low-risk disease. The EAU states that “extended LND should be performed in intermediate-risk, localized PCa if the estimated risk for positive lymph nodes exceeds 5%, as well as in high-risk cases. In these circumstances, the estimated risk for positive lymph nodes is 15%–40%. Limited LND should no longer be performed, because it misses at least half the nodes involved.” There are several nomograms available to predict the likelihood of LNI based on preoperative characteristics; these include the Partin tables, the Kattan nomogram, and the Briganti nomogram. Older nomograms for predicting the probability of LNI were based on the more limited PLND and thus underestimate the risk of LNI. The authors suggest that an individualized risk assessment be made in each case, and when the decision is made to perform a PLND, it should be an ePLND.
Surgical margins
Positive surgical margins (PSM) are reported to occur in 11% to 38% of cases and can be influenced by the surgeon. Although PSM can result from extensive cancer for which complete resection is not possible, PSM also occur because of technical error (eg, capsular incision) or inaccurate selection of patients for nerve-sparing techniques. Nomograms are commonly used to predict the probability of extracapsular extension to assist the surgeon in determining when to perform “wide-field” cavernosal nerve resection rather than nerve sparing in an attempt to reduce PSM. Several studies have reported PSM to be independently associated with biochemical recurrence after RP. Furthermore, PSM are associated with PCa-specific mortality (PCSM) in some, but not all large series. In sum, it is incumbent on the surgeon to make every effort to optimize surgical technique and patient selection to achieve negative surgical margins.
Nerve grafts have been proposed as an option for men undergoing wide-field resection, using sural, genitofemoral, or ilioinguinal nerve grafts. Several studies have suggested a beneficial effect from the grafts. In the only randomized study performed, there was no difference in erectile dysfunction between those with and without nerve grafts, although the study was underpowered, there was poor patient compliance, and 67% of controls analyzed were potent, a number much higher than expected. At present, therefore, the role of nerve grafting in PCa remains unclear.
Low-risk prostate cancer
Definition of Low-Risk Prostate Cancer
Risk stratification for patients with localized PCa includes PSA, Gleason, and T-stage data. The American Urological Association (AUA), the EAU, and the NCCN all define low-risk disease as PSA less than 10 AND Gleason 6 or less AND T1c/T2a. The NCCN also define a “very low risk disease” when all the following criteria are met: PSA less than 10, T1c, Gleason 6 or less, fewer than 3 biopsy cores positive, 50% or less cancer in any core, and PSA density less than 0.15 ng/mL/g.
Outcomes for Low-Risk Disease Following Radical Prostatectomy
Cancer-specific survival for men with low-risk disease is excellent, and approximately 40% of newly diagnosed PCa are thought to meet the definition of low risk. Stephenson and colleagues evaluated the long-term PCSM in a cohort of 11,521 men treated with RP with the finding that only 3 of 9557 men with organ-confined, Gleason-6 PCa died of PCa, further supporting the excellent long-term outcomes of RP in the low-risk subgroups. In an observational cohort of men diagnosed in Sweden from 1997 to 2002, 2686 men younger than 70 years were diagnosed with low-risk PCa (T1c, Gleason 6, PSA<10). After a median follow-up of longer than 8 years, the PCSM for those under surveillance (n = 1079) was 2.4% and was similar to those treated with RP.
RP has been shown to improve disease-specific survival in comparison with watchful waiting in randomized studies. The Scandinavian Prostate Cancer Group Study randomized 695 men to either RP or watchful waiting for localized PCa. RP was associated with a 46% reduction in PCSM (hazard ratio [HR] 0.54, 95% confidence interval [CI] 0.36–0.88) with an absolute risk reduction of 5.0%. The survival difference was most significant in men younger than 65 years. However, important considerations from this study include that only 5% of the cancer was identified by screening, 70% presented with clinical T2, 40% had a PSA greater than 10, and the analysis included men with low-, intermediate-, and high-risk disease. Concerns were raised as to whether these data on men diagnosed from 1989 to 1999 were applicable to the current state of a highly screened population and men with low-risk disease.
In 2012, data from the Prostate Cancer Intervention Versus Observation Trial (PIVOT) were published, comparing RP with watchful waiting in a highly screened population. The study enrolled 731 men, 296 of whom had low-risk disease (148 in each arm). After a median follow-up of 10 years, the cumulative PCSM was 2.7% in the watchful-waiting arm and 4.1% in the RP arm (HR 1.5, 95% CI 0.4–5.2). These data suggest that for men with low-risk disease, the 10-year risk of PCSM is low and not different between observation and treatment. However, longer follow-up out to 20 to 30 years may see a survival advantage for RP over active surveillance/watchful waiting. Regardless, treating physicians need to be aware of these excellent disease-specific survival numbers, and carefully select patients with low-risk disease who may benefit from active treatment and strong consideration for active surveillance given for most of these individuals.
High-risk prostate cancer
Definition of High-Risk Prostate Cancer
Unlike low-risk PCa, whereby an individual must have all 3 factors (PSA, Gleason score, and tumor stage) in the lowest categories, high-risk disease is defined by fulfilling any of the 3 factors in the highest category. The AUA, EAU, and NCCN all share the same PSA (>20) and Gleason (8–10) cutoffs, but differ slightly in the T-stage cutoff for high risk. The AUA guidelines consider T2c lesions to be high risk, whereas both the NCCN and EAU consider T3a to be high risk. Both the NCCN and EAU further define a “very high risk” subgroup with T3b to T4 disease. Although PSA screening has led to a significant decrease in those who meet high-risk criteria by either PSA level or tumor stage (ie, more tumors diagnosed as T1c with PSA at low levels), patients with high-risk disease continue to comprise approximately 25% of those with localized disease (down from >40% in the 1990s; Fig. 3 ). Thus, management of these patients continues to be an active part of PCa today.
Outcomes for High-Risk Disease Following Radical Prostatectomy
Recurrence rates for high-risk PCa following any treatment, including RP, are high (approximately 50%), and Fig. 4 shows disease-free survival curves from several different studies published in the AUA Clinical Guidelines.
Although recurrence is common the outcome is excellent after RP, with multiple large series ( Table 1 ) demonstrating approximately 90% 10-year disease-specific survival.
| Authors, Ref. Year | No. of Patients | 10-y Recurrence-Free Survival (%) | 10-y Disease-Free Survival (%) |
|---|---|---|---|
| Briganti et al, 2012 | 1366 | 54 | 91 |
| Stephenson et al, 2009 | 1962 | n/a | 92 |
| Boorjian et al, 2011 | 1238 | n/a | 92 |
| Ward et al, 2005 | 841 | 73 | 90 |
| Yossepowitch et al, 2008 | 1359 | n/a | 93 |
Further, RP is superior to observation in men with high-risk disease, as illustrated in the PIVOT study of RP versus observation for men with localized PCa. Men with high-risk PCa had a 60% reduction in PCSM with RP when compared with observation (HR 0.4, 95% CI 0.2–1.0). Although any of the 3 factors can make a person “high risk,” several studies have shown that having more than 1 factor is associated with worse disease-specific outcomes than having a single factor. Of note, more accurate pathologic staging after RP results in both downgrading and downstaging. For example, downstaging can occur in up to 30% of cases and downgrading in those with disease of Gleason score 8 to 10 occurs in 29% to 56% of cases. In a recent review of 380 patients with biopsy-proven disease of Gleason 8 to 10, downgrading to Gleason 7 or less occurred in 45% of patients. If tertiary pattern 5 (TP5) was considered in the pathologic grade, the downgrading occurred in only 26% of cases.
TP5 disease requires special mention, as it is not addressed in the risk strata based on biopsy. The Gleason scoring system for PCa assigns a grade to the 2 predominant patterns to yield the combined Gleason score. A third (tertiary) pattern is present in 2.3% to 48% of cases of RP specimens (by convention, a tertiary pattern is not given on biopsy specimens). The presence of TP5 in RP specimens with Gleason-7 carcinomas is associated with an increased risk of biochemical failure compared with those carcinomas of Gleason 7 without TP5. Whereas pathologic Gleason-7 disease is one criterion of the intermediate-risk group, the finding of TP5 disease represents substantially increased rates of recurrence, similar to rates associated with conventionally defined high-risk disease. Thus, finding TP5 has significant clinical consequences for post-RP patient management.
Neoadjuvant therapy before radical prostatectomy
Several studies have explored the use of neoadjuvant androgen deprivation therapy (ADT) before prostatectomy. The initial results were encouraging, with improvements in several pathologic characteristics. In a meta-analysis, neoadjuvant ADT was associated with an increased likelihood of organ-confined disease (relative risk [RR] 1.6, 95% CI 1.4–2.0), and a reduction in risk in positive margins (RR 0.5, 95% CI 0.4–0.6) and lymph node involvement (RR 0.7, 95% CI 0.5–0.9). However, there was no improvement in disease-specific outcomes with similar rates of biochemical recurrence and PCa-specific survival.
Several phase II studies of neoadjuvant chemotherapy before RP have been performed. Many of these studies have used docetaxel with or without ADT. Additional studies have combined other agents with docetaxel (eg, estramustine, mitoxantrone). Several phase II studies of novel agents and novel combinations of ADT are ongoing, including maximal androgen blockade with a combination of goserelin, bicalutamide, ketoconazole, and dutasteride ( NCT00298155 ). CALGB 90203 is a phase III study of neoadjuvant docetaxel with ADT followed by RP versus RP in patients with high-risk PCa. The study’s primary outcome is 3-year biochemical progression-free survival, and it is anticipated to end in 2018. At present, neoadjuvant therapy before RP is not the standard of care; however, the authors encourage increased access to neoadjuvant clinical trials for patients with high-risk PCa undergoing RP.
Is radical prostatectomy the preferred treatment for high-risk prostate cancer?
Historically, many patients with high-risk disease were directed toward radiation therapy, owing in part to concerns over the risk of micrometastatic disease, along with level I data from radiation studies showing improved disease-specific survival with prolonged ADT concurrent with radiation therapy in comparison with radiation therapy alone. Reports of excellent long-term disease-specific survival in men with high-risk PCa following RP has led increasingly to a reevaluation of the important role of RP in these men, with the argument for local control with RP serving as the first step in multimodal therapy. Although there have been no randomized studies comparing RP with radiation therapy, recent studies have suggested that RP may offer some disease-specific survival advantages over radiation ( Table 2 ). In one study with case-mix adjustment, 1318 men undergoing RP (of whom only 7% received either adjuvant radiation or ADT) were compared with 1062 men treated with 81 to 86.4 cGy with external-beam radiation therapy (EBRT) at a high-volume cancer center, including 409 total patients with high-risk disease. The 8-year actuarial probability of PCSM in the high-risk group was 9.5% (95% CI 4.9–17.9) for radiation and 3.8 (95% CI 1.2–11.5) for RP. In the multivariate analysis that included all patients, the RP had a 65% reduction in risk of distant metastasis compared with the radiation group (HR 0.35, 95% CI 0.19–0.63; P = .001). In a second study from CaPSURE, compared with surgery, radiation treatment was associated with a 2-fold increased risk of PCSM in a multivariate model including all-risk patients (HR 2.21, 95% CI 1.50–3.24; P <.001). A study of 6485 men undergoing RP and 2264 men treated with EBRT from 2 major PCa centers used propensity-score analysis, and found that although the 10-year PCSM rates were low in both groups, there was a 50% increased risk of PCSM with EBRT in comparison with RP (95% CI 1.0–2.3). Finally, a propensity-score–matched analysis of 40,890 men using Surveillance Epidemiology and End Results (SEER)-Medicare data found similar findings with 10-year PCSM rates of 6.8% and 11.5% in the RP and EBRT groups, respectively (multivariate HR 1.7, 95% CI 1.5–1.9). It should be noted, however, that these are not randomized trials, and selection bias is likely present. Furthermore, few men in the radiation group received prolonged (2–3 years) ADT for which randomized studies show an improvement in disease-specific survival. Finally, there were few salvage treatments for the radiation-failure group, and there were a limited number of events.
| Authors, Ref. Year | Total No. of Patients (No. with High-Risk PCa) | Notes on EBRT | 10-y Prostate Cancer–Specific Mortality Rate (95% CI) in High-Risk Patients | Risk of Mortality with XRT (Compared with RP) | ||
|---|---|---|---|---|---|---|
| RP | EBRT | RP | EBRT | |||
| Abdollah et al, 2012 | 20445 (7442) a | 20445 (7442) | cGy n/a 9% ADT | 6.8 (5.7–7.8) | 11.5 (10.2–12.9) | 1.7 (1.5–1.9) |
| Cooperberg et al, 2010 | 5052 (328) | 1143 (279) | cGy n/a 51% ADT | n/a | n/a | 1.6 (1.1–2.5) |
| Kibel et al, 2012 | 6485 (528) | 2264 (676) | 70–80 cGy 34% ADT | 1.8 (1.6–2.1) b | 2.9 (2.6–3.3) b | 1.5 (1.0–2.3) |
| Zelefsky et al, 2010 | 1318 (n/a) c | 1062 (n/a) c | 81–84.4 cGy 56% <6 mo ADT | 3.8 (1.2–11.5) d | 9.5 (4.9–17.9) d | 2.9 (1.7–5.0) e |
a Propensity-score–matched cohort analysis from Surveillance Epidemiology and End Results (SEER)-Medicare.
b PCa-specific mortality in all patients, not limited to high risk.
c Total number of patients (RP + EBRT) with high-risk disease was 409 in this study, but the number in each group was not provided.
d 8-year PCa-specific survival.
This potential surgical advantage is being extended to consideration in men with node-positive disease. Whereas previously it was common to send suspicious lymph nodes for frozen section with plans to abort the RP in the setting of positive lymph nodes, there are now emerging data for RP even in the face of clinically positive nodes. Two retrospective studies compared outcomes in patients based on findings on frozen section at the time of planned prostatectomy. The decision for proceeding with RP despite positive nodes was based on physician judgment and patient preferences. In both studies, the 10-year cancer-specific survival was higher in those treated with RP (86% and 76% ) compared with those who had RP aborted (40% and 46% ). In both studies, aborted RP was associated with a greater than 2-fold increased risk of PCSM compared with those undergoing RP (HR 2.1, 95% CI 1.1–4.1 ; and HR 2.0, 95% CI 1.6–2.6 ). Although nonrandomized and unmeasured confounding in part explains the observed differences in outcome, these findings support ongoing work in the role of tumor debulking and multimodal therapy for high-risk PCa.
Alternative Surgical Therapies
Outcomes of primary high-intensity focused ultrasound and cryoablation are highly varied among published reports ( Tables 3 and 4 ). Much of this variability may arise from differences in patient selection, length of follow-up, and inconsistencies in the definition used for failure. Posttreatment biopsies and PSA values have been the most common methods for determining failure after treatment. However, inconsistency in the definition of PSA failure has led to some series publishing results using multiple definitions of failure within the same published report. This inconsistency results in variable failure rates, even within the same cohort of patients, and hampers any conclusions regarding the efficacy of these ablative procedures.
| Authors, Ref. Year | No. of Patients | Follow-Up | Definition of Failure | Overall Recurrence-Free Survival (%) | Recurrence-Free Survival (%) in Low-Risk Patients | Recurrence-Free Survival (%) in High-Risk Patients |
|---|---|---|---|---|---|---|
| Levy & Jones, , a 2011 | 2685 COLD Registry | Median = 60 mo | Nadir + 2 ng/mL | — | 89 (5 y) | 64 (5 y) |
| Ward & Jones, , b 2011 | 1160 COLD Registry | Mean = 21.1 mo | 3 consecutive PSA rises occurring 6 mo after therapy | 75.7 (36 mo) | — | — |
| Cheetham et al, 2010 | 25 Primary (76 total) | Median = 10.1 y | Nadir + 2 ng/mL | 51.2 (of the 43 men still alive at 10 y) | — | — |
| Donnelly et al, , c 2010 | 122 | Median = 100 mo | Nadir + 2 ng/mL or radiologic evidence of disease or additional therapy | 75 (5 y) | — | — |
| Ko et al, , d 2010 | 33 high-risk | Median = 61 mo | Nadir + 2 ng/mL | 97 (36 mo) | — | 97 (36 mo) |
| Truesdale et al, 2010 | 77 | Median = 24 mo | Nadir + 2 ng/mL | 72.7 (at last follow-up) | — | — |
| Chin et al, , d 2008 | 33 high-risk | Mean = 37 mo | 3 consecutive PSA rises | 13 (4 y) | — | 13 (4 y) |
| Cohen et al, 2008 | 370 | Median = 12. 55 y | Nadir + 2 ng/mL | 62.36 (10 y) | 80.56 (10 y) | 45.54 (10 y) |
| Jones et al, 2008 | 1198 COLD Registry | Mean = 24.4 mo | Nadir + 2 ng/mL | 72.9 (5 y) | 91.1 (5 y) | 62.2 (5 y) |
| Onik et al, , b 2008 | 48 | Mean = 4.5 y | 3 consecutive PSA rises | 94 (2 y) | — | — |
| Ellis et al, 2007 | 416 | Mean = 20.4 mo | 3 consecutive PSA rises with a final value >1.0 ng/mL | 79.6 (4 y) | 83.6 (4 y) | 69.1 (4 y) |
| Polascik et al, 2007 | 50 | Median = 18 mo | PSA ≥0.5 ng/mL | 90 (at last follow-up) | — | — |
| Bahn et al, , b 2006 | 31 | Mean = 70 mo | 3 consecutive PSA rises | 92.9 (at last follow-up) | — | — |
| Prepelica et al, 2005 | 65 high-risk | Median = 35 mo | 3 consecutive PSA rises | 81.7 (6 y) | — | 81.7 (6 y) |
| Aus et al, 2002 | 54 | Median = 58.5 mo | PSA >1 ng/mL or positive prostate biopsy | 38.9 (58.5 mo) | — | — |
| Bahn et al, 2002 | 590 | Mean = 5.43 y | PSA ≥0.5 ng/mL | 62 (7 y) | 61 (7 y) | 61 (7 y) |
| Donnelly et al, 2002 | 76 | Median = 60.8 mo | PSA >1.0 ng/mL | — | 75 (5 y) | 76 (5 y) |
| Long et al, 2001 | 975 | Median = 24 mo | PSA <1 ng/mL | — | 76 (5 y) | 41 (5 y) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




