Low risk
Stage I endometrioid, grade I–II, <50 % myometrial invasion, LVSI – ve
Intermediate risk
Stage I endometrioid, grade I–II, > 50 % myometrial invasion, LVSI – ve
High-intermediate risk
Stage I endometrioid, grade III, <50 % myometrial invasion, regardless of LVSI status
Stage I endometrioid, grade I–II, LVSI +ve, regardless of depth of invasion
High risk
Stage I endometrioid, grade III, >50 % myometrial invasion, regardless of LVSI status
Stage II
Stage I with non-endometrioid histology
Patients with low-risk endometrial cancer have a low risk of lymph node involvement and do not benefit with systematic lymphadenectomy, and hence it is not routinely recommended in them [25, 35, 36].
Patients with intermediate-, high-intermediate- and high-risk endometrial cancer have a higher probability of having extrauterine disease and also have demonstrated survival benefit with systematic lymphadenectomy. Hence, a comprehensive pelvic and para-aortic lymphadenectomy is recommended in them for staging and therapeutic planning purposes [25].
Extent of Lymphadenectomy
In published literature, the extent of lymphadenectomy for endometrial cancer has been extremely variable, ranging from no lymphadenectomy to pelvic and/or para-aortic lymph node sampling to a comprehensive pelvic and para-aortic lymphadenectomy. Although there is no standard definition of “optimum lymphadenectomy” for endometrial cancer, it is clear that lymph node sampling has a low sensitivity for detecting lymph node metastases, since para-aortic lymph nodes may be involved in the absence of positive pelvic nodes [10].
The question of the optimal extent of lymphadenectomy was answered in a retrospective study of 281 patients with endometrial cancer who underwent comprehensive pelvic and para-aortic lymphadenectomy. Twenty-two percent of patients with high-risk endometrial cancer had lymph node metastases – 51 % of these had metastases in both pelvic and para-aortic nodes, 33 % had positive pelvic nodes only, while 16 % had isolated positive para-aortic nodes in the absence of metastatic pelvic nodes, with majority of patients with para-aortic metastatic nodes (77 %) having positive nodes above the level of inferior mesenteric artery [37]. On the other hand, they also found that patients with low-risk disease had no lymph node metastases and did not benefit from a systematic lymphadenectomy. Similar findings have been reported by other authors [38]. This suggests that para-aortic nodes should be removed whenever lymphadenectomy is indicated and that it is essential to extend the upper limit of lymphadenectomy to the level of renal vessels.
There are two ways to judge the adequacy of lymphadenectomy. The more accurate way is to perform a complete pelvic and para-aortic lymphadenectomy as per the anatomic templates. The other is to measure the lymph node count in the surgical specimen, which is a surrogate marker for adequacy of lymph node dissection (it has been shown that patients with more than 10–12 nodes removed during lymphadenectomy have an improved survival). In the collated data of 16,995 patients of endometrial cancer from two randomized controlled trials and seven observational studies, Kim et al. demonstrated an improved overall survival with systematic lymphadenectomy (i.e. removal of more than 10–11 nodes) in patients with intermediate- and high-risk endometrial cancer but limited survival benefit in low-risk patients [39–41]. Based on this, lymph node counts have become a surrogate for adequacy of lymphadenectomy with the recommendation that more than ten nodes should be removed in an adequate lymphadenectomy [42, 43].
Does Lymphadenectomy Improve Survival?
Two randomized studies [44, 45] comparing systematic pelvic lymphadenectomy to no lymphadenectomy in the surgical management of patients with endometrial cancer demonstrated that lymphadenectomy improved surgical staging but had no impact on overall survival.
However, despite the randomized trials showing no survival benefit with comprehensive surgical staging, controversy still exists regarding the role of lymphadenectomy, mainly due to the criticisms of the ASTEC trial [46]. This trial was criticized for a faulty trial design, a high rate of crossover to radiation therapy and selection bias. Neither trial included para-aortic lymphadenectomy, and the ASTEC trial also had low lymph nodal counts. This omission of para-aortic lymphadenectomy may have negated the therapeutic effect of lymphadenectomy since more than half of the patients with positive pelvic nodes have para-aortic nodal metastases, and about 10 % of lymph node metastases occur exclusively in the para-aortic region without pelvic lymph nodal involvement as shown by the sentinel node studies [47]. Removal of para-aortic lymph nodes could probably explain the significant effect of para-aortic lymphadenectomy as shown by Todo et al. [48]. They analyzed their study of intermediate and high-risk patients who underwent surgery with pelvic lymphadenectomy with or without para-aortic lymphadenectomy. Those who had para-aortic lymphadenectomy had a survival benefit as compared to those who did not [48]. The findings of this SEPAL study, similar to the ASTEC trial, suggested that the survival effect of lymphadenectomy is rather limited in low-risk patients but is quite substantial in the intermediate- or high-risk patients, with reduction in the risk of death (HR 0.44, p < 0.0001). In the ASTEC trial, patients were secondarily randomized to radiation therapy based on uterine pathology only without considering the nodal status, leaving some patients with metastatic nodes with no adjuvant therapy. The clinical benefit of triage to adjuvant therapy was obscured as 50 % of patients with lymph node metastases were randomized to no adjuvant therapy. Besides, the lymphadenectomy versus no lymphadenectomy arms were unbalanced in terms of high-risk criteria, with the lymphadenectomy arm having a greater percentage of patients with high-risk histology, high-grade tumours, presence of lymphovascular invasion and deep myometrial invasion. Lastly, this trial did not address the issue of benefit from para-aortic lymphadenectomy as patients underwent para-aortic node palpation with selective sampling rather than systematic lymphadenectomy.
Retrospective data suggests that patients undergoing systematic lymphadenectomy had improved survival over those who had limited or no lymphadenectomy [43]. An analysis of 42,184 patients from the SEER database revealed that systematic lymphadenectomy was associated with overall and cancer-specific survival benefit (HR 0.81 and 0.78, respectively), and removal of more than 11 nodes was associated with HRs of 0.74 and 0.69, respectively [49]. Although statistically significant, the retrospective nature of the data was subject to selection bias and stage migration. Trimble et al., using a large national database, reported benefit with lymphadenectomy in grade III tumours only [50].
Sentinel Node Mapping
The lymphatic drainage of uterus is complex, with several anatomical areas at risk for metastases. The sentinel node is defined as the first node in the lymphatic basin that receives the lymphatic flow. If the SLN is negative for metastatic disease, other nodes in the template are expected to be free of disease involvement. The advantage of SLN biopsy is the potential for improved diagnostic accuracy with use of ultrastaging while lowering morbidity [51, 52]. Sentinel node biopsy in particular has the advantage of limiting the risk of lymphedema, which is seen in 6–38 % of patients following pelvic lymphadenectomy [53, 54].
Sentinel node mapping for uterine cancer was first described by Burke et al. [55]. They reported on 15 patients who had SLN mapping followed by complete pelvic and para-aortic lymphadenectomy. They reported an overall SLN detection rate of 67 %. Four patients had positive lymph nodes – two of these were detected by SLN mapping with blue dye, one had a positive non-sentinel node and one had bulky nodes without dye uptake. Khoury-Collado et al. (2011) could successfully identity the sentinel node in 84 % of the cases in their study of 266 cases of endometrial cancer, with 12 % incidence of metastatic nodes and 3 % metastatic nodes being confirmed by immunohistochemistry [56]. Ballester et al. [51] in their multicentre SENTI-ENDO trial showed that 10 % of low-risk and 15 % of intermediate-risk patients were upstaged using the sentinel node technique [51].
The greatest challenge in using the SLN technique in endometrial cancer is to identify the optimum injection site that properly represents the drainage of the tumour. Most large series till date have used cervix as the injection site. In recent times, endometrial site of injection using the hysteroscopic, ultrasound-guided, laparoscopic and open approaches has been investigated. Hysteroscopy allows injection of the tracer in the mucosal space just around the tumour and at least conceptually should be the best way to delineate the drainage of the tumour. Hysteroscopic injection also allows a complete detection of the drainage of the uterine corpus directed to both pelvic and para-aortic nodes, thereby decreasing the false-negative rates. The first report of hysteroscopy-guided SLN technique by Nilkur et al. [47] showed a detection rate of 82 % with no false negatives. Subsequently, Maccauro et al. [57] and Raspagliesi et al. [58] reported a detection rate of 100 % with no false negatives [57, 58]. Presently, however, there is no definite evidence that these technically more demanding injection approaches have a definite benefit over cervix as the injection site [59].
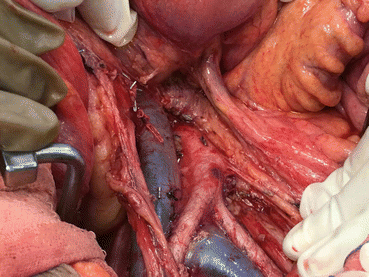

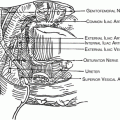
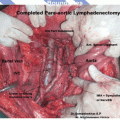
Para Aortic Lymphadenectomy
References
1.
2.
Connor JP et al. Computed tomography in endometrial carcinoma. Obstet Gynecol. 2000;95(5):692–6.PubMed
3.
4.
5.
6.
7.
Bansal N et al. The utility and cost-effectiveness of pre-operative computed tomography for patients with uterine malignancies. Gynecol Oncol. 2008;111(2):208–12.CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree