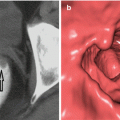
Morphology: flat, hard to see, sessile > 15 mm, laterally spreading, villous, unsmooth surface, short and thick pedicle
Diameter: <1.5 cm, or > 3 cm
Multiplicity (>3)
Localization: right colon or cecum, ileocecal valve, entrance of the appendix, behind the fold
Polyps with disadvantageous size or location have difficulties which prevent polyp removal, cause risk of bleeding (sooner or later), obstruct the lumen completely or near completely, and make endoloop or snare placement impossible. Hence the term is used for irremovable large polyps. In the case of sessile polyps, it is difficult to remove the polyp completely. Therefore, large sessile colonic polyps are usually surgically managed in spite of the morbidity and potential for mortality. Endoscopic mucosal resection (EMR) is used as an alternative method for sessile colonic polyps. In this procedure, by injecting a submucosal saline and colorant (methylene blue or indigo carmine) mixture, mucosa is separated from submucosa. Afterwards, using snare in piecemeal fashion, the whole sessile lesion is resected with partial normal mucosa [6, 7]. This procedure requires a standardized definition (Paris classification, granularity, pit tarren) of the polyp [8]. The Paris classification is an endoscopic morphology system for gross polyps [9]. Protruding type polypoid lesions are classified as pedunculated (type 0-Ip), sessile (0-Is), or mixed type (0-Ips) whereas the superficial types (higher than 2.5 mm) are classified as superficial elevated (type 0-IIa), flat (0-IIb), shallow depressed (0-IIc), or excavated (0-III). Superficial polypoid lesions (2.5 mm in height, 10 mm in size) that typically extend laterally along the colonic wall, called laterally spreading tumor (LST), are either adenomatous or serrated (with a saw tooth histological architecture) and have a malignant potential for early invasion. Based on their gross endoscopic appearance, LSTs are classified as LST-granular (G) type and LST-nongranular (NG) type. The former has an uneven nodular surface whereas the latter has a smooth surface.
The pit pattern classification for colonic polyps, developed by Kudo and colleagues in 1996, is based on the appearance of pit patterns in the colonic mucosa overlying the polyp lesion and consists of 6 subtypes: type I: round (normal); type II: star-like (stellar) or papillary (hyperplasic or serrated polyp); type III L: tubular or roundish, larger than normal (tubular adenoma); type III S: tubular or roundish pits, smaller than normal (adenoma—possible high-grade dysplasia); type IV: sulcus-, branch -, or gyrus-like (villous adenoma); and type V: irregular (Vi) or nonstructural (Vn) (invasive cancer). By this means, the malignancy and invasion risk of the polyp can be calculated before suggesting surgical resection to the patient. Disadvantageous polyp location for EMR, ileocecal invasion by the polyp, and previous resection attempts of endoscopist may lead to unsuccessful procedures. Fibrosis after the first procedure prevents the separation of mucosa and submucosa, leading to an unsuccessful EMR. Such cases are suitable candidates for surgical resection. Piecemeal polypectomy of pedunculated large polyps (larger than 3 cm) may have negative consequences in pathological terms. Remaining polyp pieces in the colonic mucosa fail to receive a pathological diagnosis. Furthermore, piecemeal removal carries a high risk for bleeding for large polyps. Endoclip-assisted resection of large pedunculated colon polyps could be considered a more suitable technique [9–11]. In this technique, multiple clips are placed on the stem of the polyp. Afterwards, the polyp is removed from its stem using a needle cautery. Clips and electrosurgery equipment proper for this procedure have recently being developed.
Combined endoscopic and laparoscopic surgery (CELS) is defined for cases in which endoscopic polypectomy cannot be performed due to localization. In this procedure, the colon is seen using the laparoscopy to help with endoscopic polypectomy manipulation. When polyps with a benign appearance cannot be resected by using endoscopy, CELS may be a better alternative than bowel resection due to its reduced morbidity. For polyps that are not resectable with standard colonoscopy, a combination of endoscopic and laparoscopic surgery may be considered as an option [12].
Surgery for Advanced Adenomas with Malignant Potential or Malign Polyps
We performed polypectomy, and the results came back malignant. What should we do? The answer requires sufficient data.
Should all the malign polyps be approached with surgical treatment? When the malign polyps of the low risk-group undergo surgery, a small health benefit comes at a great cost. For this reason, low-risk malignant polyps can be followed after polypectomy. The only necessity is a meticulous histopathological examination. Furthermore, polyps to be followed or to be referred to surgery must be marked with a long-lasting dye that can be seen from the serosal surface. If the polyp cannot be located during surgery, intraoperative colonoscopy could be helpful.
Histopathological examination of the polyp determines the way of management. Treatment modalities change starting from the term “advanced adenoma.”
Advanced adenomas are larger than 1 cm, have villous architecture, display high-degree dysplasia, and are highly susceptible for CRC [13–18].
Single cell infiltration inside the mucosa, small gland proliferation, or visible enlargement in the back-to-back cribriform-pattern glands and their invasion of lamina propria or muscularis mucosa are defined as intramucosal adenocarcinoma. Significant invasion in lamina propria accompanied by desmoplasia is important for differentiating between intramucosal adenocarcinoma and high-grade dysplasia [19]. Since no metastases are present in high-grade dysplasia and intramucosal adenocarcinoma, polypectomy and follow-up surveillance is suggested. The prerequisite for follow-up is complete resection of the lesion within solid safety boundaries [20]. Morbidity and mortality of the colon resection can be avoided this way.
Malignant Epithelial Polyp
Malignant epithelial polyp can be defined as an adenoma with an invasive adenocarcinoma (cancer within the polyp stem, reaching beyond the muscularis mucosa) [21, 22]. It is classified as T1 in TNM and carries risk for metastasis. Carcinoma in situ (high-grade dysplasia, intraepithelial carcinoma) refers to cases where the cancer cells are limited to glandular basal membrane, and intramucosal carcinoma is a term for invasion of the lamina propria but not the muscularis mucosa [22]. There is no risk of metastasis in this phase, and it is identified as pTis in TNM system. According to the Vienna classification, it is a noninvasive high-grade dysplasia [23]. Malign polyps consist 0.2–11 % of the endoscopically resected polyps [22].
Various classification systems have been suggested for evaluating the level of invasion. The Haggit classification is based on the level of adenocarcinoma presence within the polyps (0: Limited to mucosa, 1: Limited to the head of the polyp, 2: Neck level, 3: Stem level, 4: Submucosa). Sessile polyps with an invasion are directly accepted as grade 4 [21].
Evaluation of lymphatic invasion is important for lymph node metastasis [24, 25]. Assessing lymphatic invasion may especially be difficult for areas with retraction artifact. D2-40 (podoplanin), a dye immunohistochemically specific to lymphatics, is suggested as a solution. Vascular invasion should also be considered for prognosis.
The Haggit classification suggests that the depth of invasion is the most significant parameter for prognosis and that while the submucosa invasion carries a risk for metastasis, the stem invasion do not. Kikuchi classification identifies lymph node metastasis percentages as 1–3 for sm1, 8 for sm2, and 23 for sm3 [25]. While there are studies supporting the Haggit classification [26], there are also others which indicate surgical borders to be the most important prognostic factor [20, 27].
In order to determine the prognosis and correct treatment for malignant polyps, their histological characteristics are divided into two groups: Desirable and undesirable [22, 25]. Among the desirable characteristics are grades 1 or 2, carcinoma cells further by at least 2 mm from the closest surgical border, and no presence of vascular or lymphatic invasion [22]. In addition, in terms of the depth of the invasion, it should be classified as level 1–3 in Haggit and sm1 in Kikuchi [25]. Polypectomy is sufficient for these polyps [22]. However, studies indicated 1 % recurrence level.
Undesirable histological characteristics include invasive tumor cells being closer than 2 mm to the nearest surgical border or being at the surgical border, grade 3, or lymphatic/vascular invasion [22]. In terms of invasion depth, it should be classified as level 4 in Haggit and level sm2-3 in Kikuchi [25]. Recurrence and residual disease rate have been reported to be 10–39 % for these polyps [25, 28]. Due to lymph node metastasis and residual disease risk, surgical resection is considered to be necessary for these cases [28].
NCCN guideline for malignant polyps is indicated below.


Architectural structure of the adenoma, villous predominance of the lesion, presence of high-grade dysplasia and/or intramucosal adenocarcinoma, and size of the lesion are among the parameters that should appear in the pathology report in deciding between surgical treatment and follow-up controls for adenomatous polyps. If the diagnosis is “intramucosal adenocarcinoma in the adenoma,” absence of invasive cancer in the polyp pedicle and sufficiency of surgical borders should be well documented. Presence of invasive cancer on the polyp pedicle requires surgical treatment. Atypical appearance of the damaged cells in the resection border due to cautery artifact may sometimes make evaluation impossible. For this reason, resection area should be evaluated meticulously, and information on the surgical borders should be given in the pathology report [20]. Even for the low-grade dysplastic adenomas, if the cauterized area contains adenomatous tissue, it should either be excised later or should be followed up closely. All these procedures require a permanent marking of the resected polyp area.
As suggested on the other chapter of the book, patients with FAP risk should be followed up with a sigmoidoscopy and colonoscopy, starting from their puberty. When polyps are detected, the colon and the rectum should be resected prophylactically [29]. CRC risk of AFAP patients increases after the age of 60 [30]. Primary surgical treatment suggested for those with more than 20 adenomas and a progressed adenoma which is larger than 1 cm is colectomy. Colectomy could be performed with laparoscopy or with open surgery.
Surgical Treatment
For a polyp to require surgery, it either has to be a malignant one with negative criteria (diagnosed) or it has to be large enough and positioned in a way that prevents endoscopic removal. As previously stated, the first step of laparoscopy or open surgery is to permanently mark the polyp or the resected polyp area. Malignant polyps which are not suitable for follow-up should be evaluated like an invasive cancer and resected in line with the oncological principles for the colorectal cancer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree